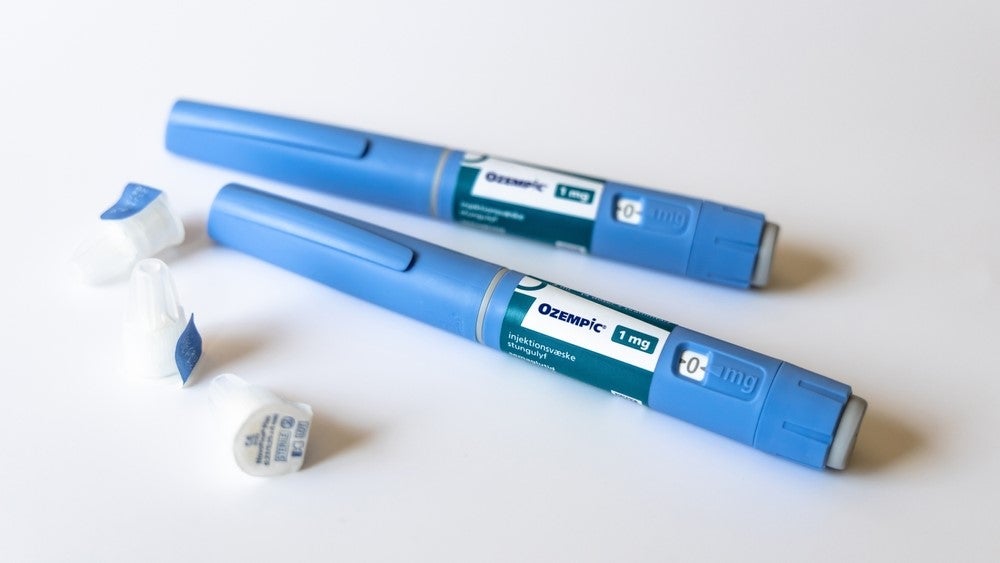
As Novo Nordisk reports record sales for its blockbuster Ozempic (semaglutide), increased alerts of counterfeit versions of the drug being sold continue to gather.
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has identified two wholesalers who were selling falsely labelled Ozempic pre-filled pens.
The agency was quick to add that all the counterfeit pens have been accounted for and ”none of the pens have been supplied to UK patients”. The MHRA also revealed that counterfeit products were imported from legitimate suppliers in Austria and Germany.
This news comes after the German Federal Institute for Drugs and Medical Devices (BfArM) announced it was investigating multiple cases of counterfeit Ozempic being sold in the country.
The agency noted that the outer packaging of the original and counterfeit drugs were identical, however, the products’ primary packaging was different making it easy to identify the fake products. BfArM also released images of original and counterfeit Ozempic in an 11 October press release.
Ozempic has been approved for treating type 2 diabetes (T2D) and is available in three doses, namely 0.5mg, 1mg, and 2mg. The drug has also been used off-label to treat obesity. Novo Nordisk reported Dkr42.7bn ($5.9bn) in global sales for Ozempic during the first half of 2023, as per the company’s Q2 financials.
In June 2023, the Danish company raised the alarm regarding the false products after counterfeit Ozempic was found in the US. In that case, the counterfeit product contained insulin glargine and caused an adverse reaction in patients since it worked as per a different mechanism of action than Ozempic.
The counterfeit Ozempic found in the UK had the same packaging as the false German products previously identified by the BfArM.
MHRA chief safety officer Dr Allison Cave stated: “Anyone who suspects that they’ve had an adverse reaction to semaglutide or any other medicinal product, are worried about its safety or effectiveness, or suspect it is not a genuine product, should report it to our Yellow Card scheme.”
Recently, Novo Nordisk stopped the Phase III trial (NCT03819153) evaluating Ozempic for the treatment of chronic kidney disease (CKD) in patients with T2D. The trial met its primary endpoint and efficacy criteria for early trial termination by delaying the decline of renal function by 50% or more. Furthermore, Ozempic reduced the risk of cardiovascular and CKD-associated mortality.