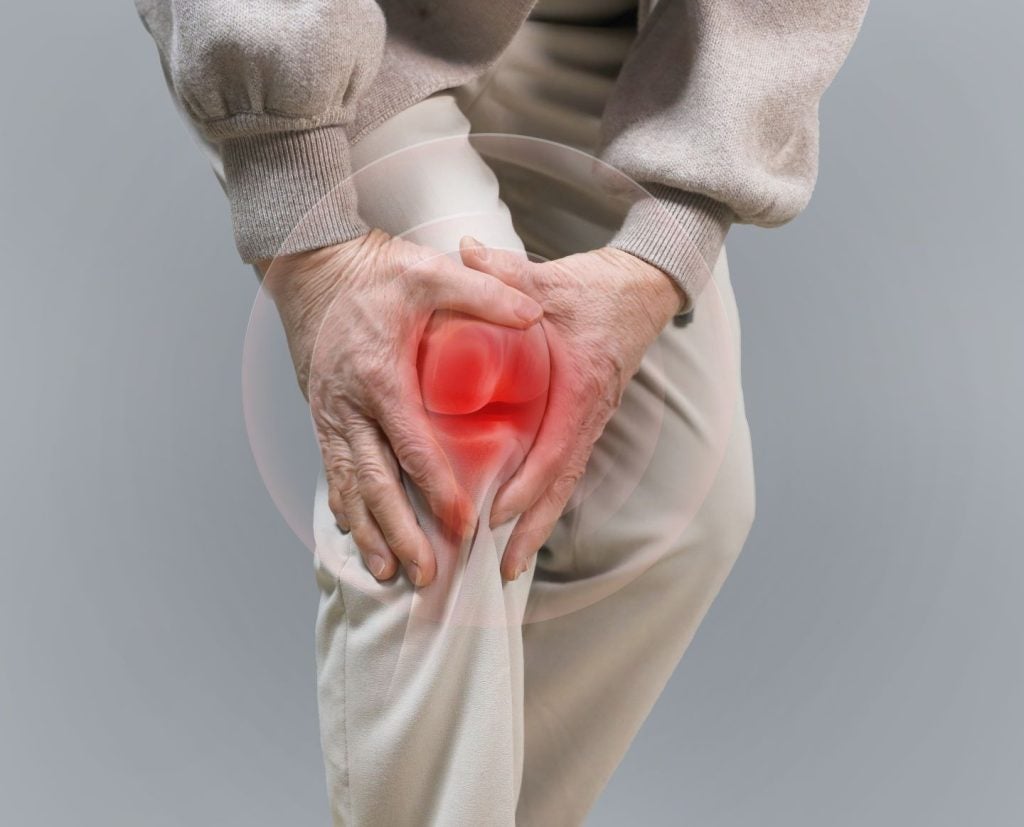
Sun Pharmaceutical Industries (Sun Pharma) and Moebius Medical have received fast track designation (FTD) from the US Food and Drug Administration (FDA) for MM-II (Large Liposomes of DPPC [dipalmitoylphosphatidylcholine] and DMPC [1,2‐dimyristoyl‐sn‐glycero‐3‐phosphocholine]), a non-opioid treatment for osteoarthritis knee pain.
Osteoarthritis is a common chronic joint disease marked by the deterioration of joint cartilage.
The FTD aims to expedite the development and review of treatments for serious conditions with unmet medical needs.
This programme will accelerate the availability of new medicines that could significantly improve patient outcomes.
Sun Pharma and Moebius will benefit from more frequent communication with the FDA and the possibility of an expedited approval process.
Moebius Medical CEO Moshe Weinstein stated: “This fast track designation, which will enable the FDA to review MM-II in an expedited manner, is an important milestone in the development of MM-II and follows our recently released Phase IIb data, which showed MM-II's potential to provide effective and durable treatment for patients with knee pain of osteoarthritis."
MM-II is based on a technology involving large liposomes composed of DPPC and DMPC, designed to reduce joint friction and wear, thereby alleviating pain.
The potential of MM-II was demonstrated in a Phase IIb clinical trial (NCT04506463), where a single 3ml intra-articular injection provided substantial pain relief compared to a placebo for up to 26 weeks.
The companies are now preparing for confirmatory Phase III clinical trials to further evaluate the efficacy and safety of MM-II.
Sun Pharma Global Development head and senior vice-president Marek Honczarenko stated: “As we enter Phase III development, we are very encouraged by the FDA's decision to grant fast track designation to MM-II and recognise its potential to fill an unmet medical need for patients suffering from osteoarthritis.”