SparingVision expects to start a new Phase I trial of its ocular gene therapy SPVN20 in H2 2024, said CEO Stéphane Boissel in an interview with Pharmaceutical Technology.
The French company is finalising clinical trial application (CTA)-enabling studies for its gene therapy, with plans to submit the CTA in 2024, added Boissel.
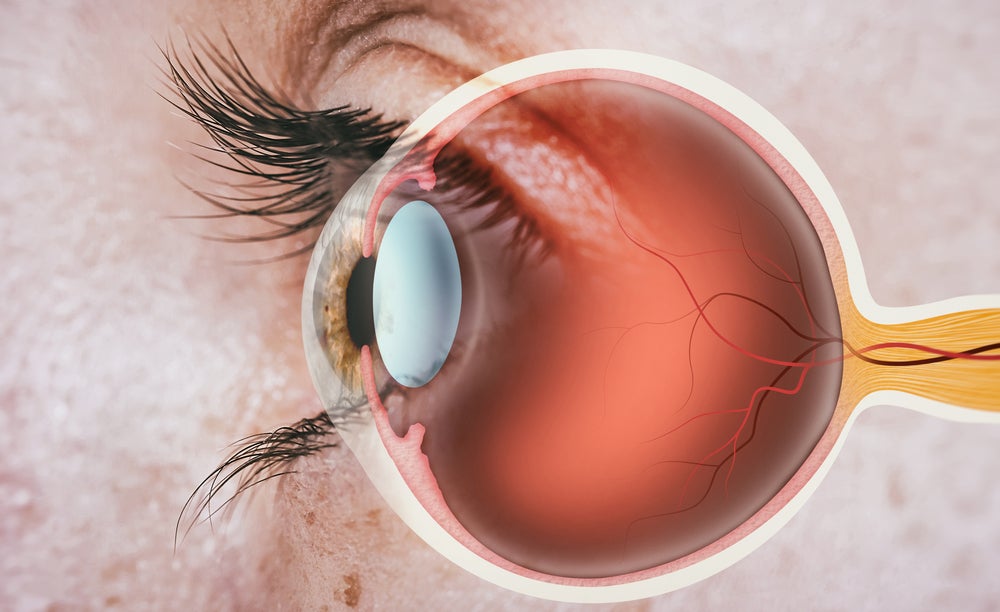
SPVN20 is an adeno-associated virus-based gene therapy that could improve visual acuity in advanced and late-stage retinitis pigmentosa patients, as per the company.
The gene therapy seeks to restore the function of viable non-functional cones by encoding for a variant of GIRK, a GPCR-activated ion channel that reactivates cone function, per an April 2021 press release. In April 2021, SparingVision acquired the biotech GAMUT Therapeutics, which also gave it access to the company’s lead candidate, which was renamed SPVN20.
The Phase I trial, named NYRVANA, will have a data readout in 2025, said Boissel. The trial will be shorter and smaller than the ongoing Phase I/II PRODYGY study (NCT05748873) of another therapy SPVN06 in stage two and three retinitis pigmentosa, explained Boissel. Although Boissel did not disclose the exact number of subjects in the trial, he said dose escalation trials usually investigate between two to three doses with three to five subjects per dose.
According to the National Eye Institute, retinitis pigmentosa is a group of rare eye disorders, which result in a gradual breakdown of retina cells, rod-cone dystrophy and vision loss. There is no available cure for the condition.

Last month, SparingVision shared positive initial safety data from the PRODYGY study’s first cohort, stating that the therapy was well-tolerated at low doses in the first cohort. SPVN06 is an adeno-associated virus-based gene therapy that consists of one neurotrophic factor RdCVF and one enzyme that slows the degeneration of cone photoreceptors that lead to blindness in patients with rod-cone dystrophies, based on the company’s website. In June 2020, the European Commission granted SPVN06 orphan drug status in inherited retinal dystrophies.
The company is now working on recruiting patients for further cohorts in PRODYGY. SparingVision expects to recruit subjects for the second cohort in early November, said Boissel. Additionally, the enrollment of participants for the third cohort will finish sometime early next year, added Boissel.
Afterwards, the company will move into the second part of the trial, which will be a control study that will recruit patients between Q1 and Q3 2024, the CEO stated. As part of this, the company expects to open up three more US-based sites, bringing the total to five trial locations, he said. SparingVision expects to have results ready from the trial in 2025, added Boissel.
SparingVision expects that its current funding secured through a past Series B will last until the second half of 2025, he added. In September 2022, the company raised €75m as part of the Series B.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.