England’s National Health Service (NHS) has reported an 18% increase in the number of people with non-diabetic hyperglycaemia – also known as pre-diabetes.
The latest National Diabetes Audit shows that 3,615,330 people registered with a GP have pre-diabetes and are at a greater risk of developing type 2 diabetes (T2D). Compared to 2023 figures, this is up by more than half a million patients.
The NHS has rolled out a range of programmes and services to curb the proportion of this cohort that are diagnosed with T2D. However, for those who still develop diabetes, a much-changed treatment landscape now exists.
Previously, metformin and other anti-hyperglycaemic medications were the only pharmaceutical options for patients with T2D. Metformin is still the first-line treatment of choice for T2D patients due to its efficacy, safety, and cost.
Now, the proliferation of glucagon-like peptide-1 receptor agonists (GLP-1RAs), in particular, has transformed diabetes care. These drugs work by mimicking the GLP-1 hormone that is normally released after eating, which stimulates various glycaemic control mechanisms.
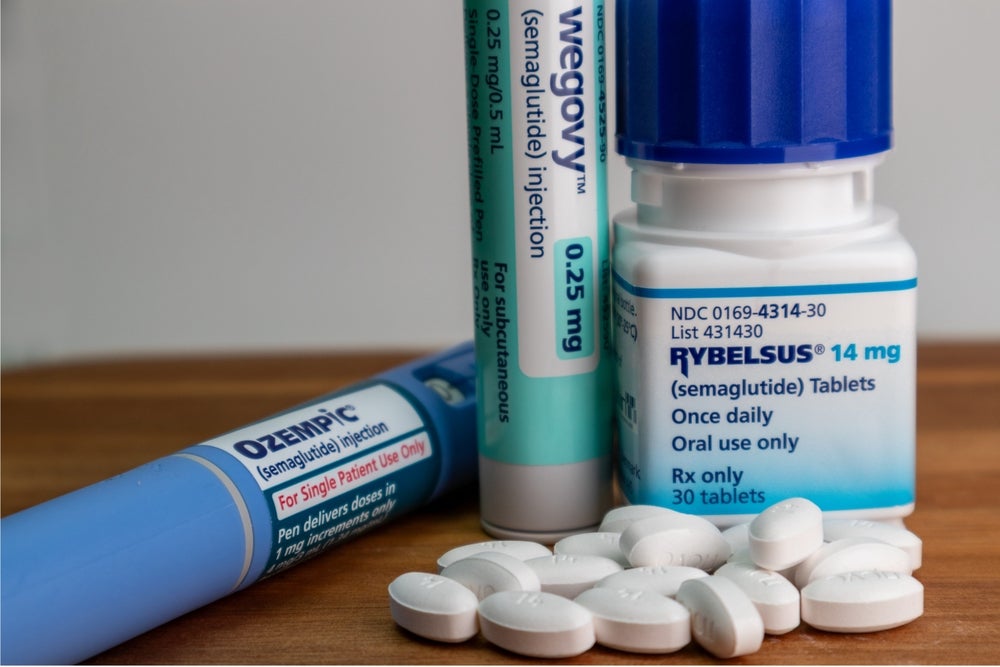
GLP-1RA options on the NHS
There are currently six GLP-1RAs available in the UK. The more popular options are Eli Lilly’s Mounjaro (tirzepatide) and Novo Nordisk’s Ozempic (semaglutide). Novo Nordisk also markets semaglutide in the oral form as Rybelsus. Other approved treatments include Eli Lilly’s Trulicity (dulaglutide), Novo Nordisk’s Victoza (liraglutide), and Sanofi’s Lyxumia (lixisenatide), all GLP-1RAs.
The sharp rise in uptake of GLP-1RAs has outpaced supply, however, leading to shortages in the NHS earlier this year. Off-label prescribing worsened the shortages, prompting guidance from a Medicines Safety Notice in March telling clinicians to only prescribe the drugs for their licensed indication.
At the same time, an increased supply of Rybelsus, and the introduction of Mounjaro in the supply chain have been highlighted by charity Diabetes UK. The shortages are likely expected to last until the end of this year, according to Douglas Twenefour, head of care at Diabetes UK.
Twenefour added: “We fully support the instruction that GLP-1 medications should not be prescribed off-label under any circumstances while there is an ongoing shortage impacting people with T2D.”
Global pharma scene
Novo Nordisk and Eli Lilly, market leaders for GLP-1RAs, are now the two biggest pharmaceutical companies in the world. Novo Nordisk has a market cap of $436bn while Eli Lilly’s market cap is $839.5bn.
Their success has meant other players in the industry have been making a push into diabetes – and more broadly weight loss – treatments. AstraZeneca received US Food and Drug Administration (FDA) approval earlier this week for its paediatric type 2 diabetes treatment Farxiga (dapagliflozin). Farxiga, marketed as Forxiga in the EU, is already approved as an adjunct to diet and exercise to improve glycaemic control in adults with T2D.
The company signed a deal worth a potential $1.85bn last November to gain an exclusive licence to Eccogene’s T2D pill ECC5004, at the time in the early stages of development.
Roche signalled its intent to join the GLP-1RA arena a month later with its acquisition of Carmot Therapeutics for $2.7bn, along with $400m in milestone payments.
Pfizer is eying the lucrative metabolic disease market but suffered a setback after its weight loss pill, lotiglipron, caused serious side effects in a Phase IIb trial, causing the company to drop its GLP-1RA candidate. Pfizer will rest its hopes on its other candidate, danuglipron, to take on competitors.