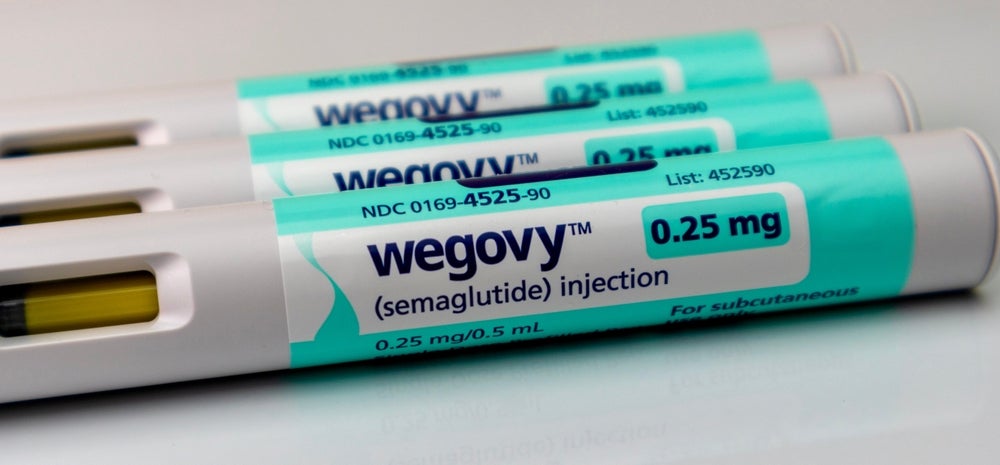
In the same week that Eli Lilly filed three lawsuits against online vendors and wellness spas making compounded versions of tirzepatide, Novo Nordisk has asked the US Food and Drug Administration (FDA) to prevent compounders from manufacturing copycat versions of its blockbuster drug semaglutide.
In the latest attempt to protect its prized diabetes and obesity treatments Ozempic and Wegovy, the Danish drugmaker has nominated semaglutide products to be included on the FDA’s Demonstrable Difficulties for Compounding (DDC) lists, as per two documents submitted to the agency. The DDC list identified drugs that are too difficult to compound due to the complexity of formulation, delivery, or dosage, amongst others.
FDA-approved drugs can be compounded under certain conditions such as if the original approved drug is in shortage and unavailable. Many patients seeking GLP-1 agonists turned to compound pharmacies as demand for Novo Nordisk’s and Eli Lilly’s popular drugs far-outpaced supply, leading to shortages of the FDA-approved products.
Novo Nordisk said the safety risks associated with compounded semaglutide are “significant”. The company outlaid key differences in formulations – such as pharmacies using synthetic semaglutide, dosing errors, and limitations in bioavailability. Novo Nordisk has requested the FDA set up an advisory committee to discuss the addition of its blockbuster drug to DDC lists.
"The nomination of semaglutide to the FDA’s DDC Lists is a significant step towards keeping people safe from unapproved and potentially harmful versions of knock-off 'semaglutide' drugs," a Novo Nordisk spokesperson told Pharmaceutical Technology.
"These drugs are inherently complex to compound safely, and the risks they pose to patient safety far outweigh any benefits. Novo Nordisk’s aim with this nomination is to ensure that patients receive only FDA-approved, safe, and effective semaglutide products," they added.
The request to the FDA is just the latest action Novo Nordisk has taken related to semaglutide compounding. The company filed nine new lawsuits in May this year against several medical spas, weight loss clinics, and pharmacies that were selling compounded drugs claiming to contain semaglutide. Novo Nordisk states that 442 cases of adverse events associated with these products have been reported to the FDA, 319 of which were classified as serious. There have also been issues of fake batches of Ozempic passing through Brazil and the UK in the past year. Eli Lilly has been taking legal action of its own against copycat producers of tirzepatide.
The FDA has previously warned those seeking compounded GLP-1 drugs of the risks, stating that “unapproved versions do not undergo FDA’s review for safety, effectiveness and quality before they are marketed”. The agency is permitting the manufacture of semaglutide and tirzepatide, despite the latter drug being removed from the FDA’s drug shortage list.
Alliance for Pharmacy Compounding’s CEO Scott Brunner did not hold back in a statement following Novo Nordisk’s request, citing “desperation” and “ridiculous claims” made by the drugmaker. Brunner said testing labs that the alliance has contacted say drug impurities are within accepted industry ranges, contradicting Novo Nordisk’s efficacy and safety shortcoming claims.
“Novo Nordisk is apparently so deeply concerned about patient safety that it’s taken them a whopping two-and-a-half years while their drug has been in shortage to conclude that the semaglutide active pharmaceutical ingredient is so demonstrably difficult for compounding pharmacies to prepare that FDA now needs to place it off-limits for compounding?” he asked.
Brunner added: “We continue to be in a prolonged era in which the finished-form FDA-approved semaglutide injection products are not available,” Brunner explained, adding that the latest attempt by Novo Nordisk to block copycat semaglutide is to “protect its revenue stream [rather] than a serious scientific argument.”
The success of Ozempic and Wegovy has catapulted Novo Nordisk to the top spot in the list of most valuable companies in Europe, with the drugmaker holding a market cap of $520bn. The drugs are forecast to rake in $23.4bn in annual revenue for Novo Nordisk by 2030, according to GlobalData’s Pharma Intelligence Center.
GlobalData is the parent company of Pharmaceutical Technology.
Note: This article was updated on 25 October to include comment from a Novo Nordisk spokesperson.
Paragraph nine was updated on 28 October to correctly characterise Brunner's statement on the test results for compounded drugs.