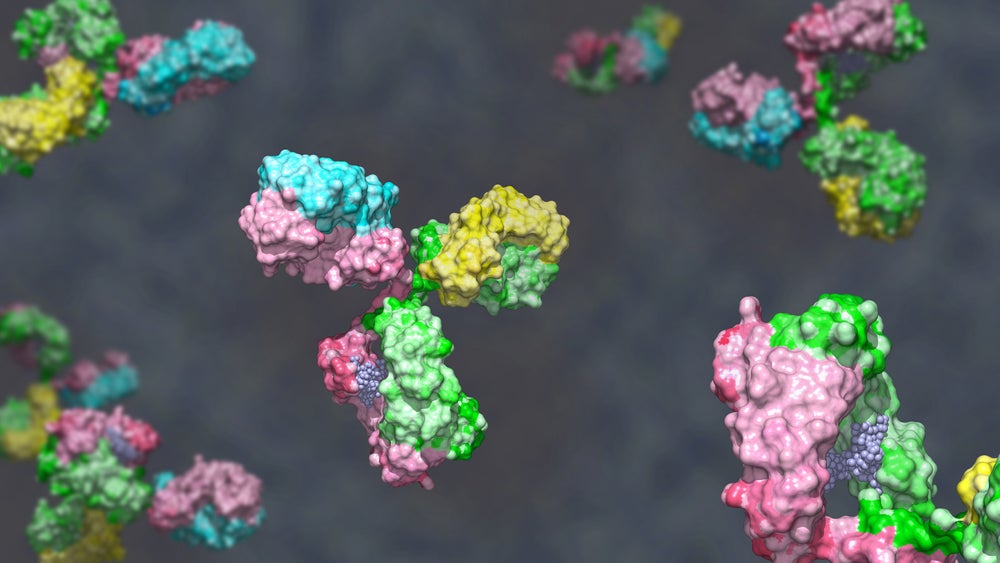
The US Food and Drug Administration (FDA) has granted an orphan drug designation to Phanes Therapeutics’ PT217 in neuroendocrine carcinoma (NEC) indication.
This is the second orphan drug designation the therapy has received from the FDA. In 2022, PT217 received the designation for the treatment of small cell lung cancer (SCLC). The orphan drug status gives sponsors tax credits from clinical trials, exemption from user fees, and a potential seven years of market exclusivity for the therapy after approval, compared to the usual five years.
PT217 is a delta-like ligand 3 (DLL3) and a cluster of differentiation 47 (CD47)-targeting bispecific antibody. Phanes is investigating the therapy in patients with advanced or refractory cancers expressing DLL3, including SCLC, extrapulmonary NECs, and large cell neuroendocrine carcinoma of the lung (LCNEC). The Phase I SKYBRIDGE trial (NCT05652686) is evaluating the safety, tolerability and pharmacokinetics of PT217 in combination with Roche’s PD-L1 therapy Tecentriq (atezolizumab).
Multiple pharmaceutical companies have invested in the development of bispecific antibody therapies. Earlier this month, Merck & Co (MSD) unveiled plans to acquire a CD3 and CD19-targeting T cell engager bispecific antibody, CN201, from Curon Biopharmaceutical. The deal is worth approximately $1.3bn in upfront and milestone-based payments. It is expected to close in Q3 this year.
In May, Johnson & Johnson (J&J) paid $1.25bn to acquire global rights for Numab Therapeutics’ IL-4R and IL-31-targeting bispecific antibody. The therapy is being evaluated as a treatment for atopic dermatitis in a Phase I trial (NCT05859724).
Other therapies in Phanes’s pipeline are a CD73-targeting monoclonal antibody PT199, a Claudin 18.2 (CLDN18.2), and the CD47-targeting bispecific antibody PT886. The company is evaluating PT199 as a combination treatment with BeiGene’s Tivimbra (tislelizumab) for solid tumours in a Phase I MORNINGSTAR trial (NCT05431270).
The bispecific antibody PT886 is being trailed in combination with MSD’s Keytruda (pembrolizumab) in a Phase I/II TWINPEAK study (NCT05482893). The trial is expected to enrol approximately 114 participants with either unresectable or metastatic gastric adenocarcinoma, gastroesophageal junction (GEJ) adenocarcinoma, and pancreatic ductal adenocarcinoma (PDAC).