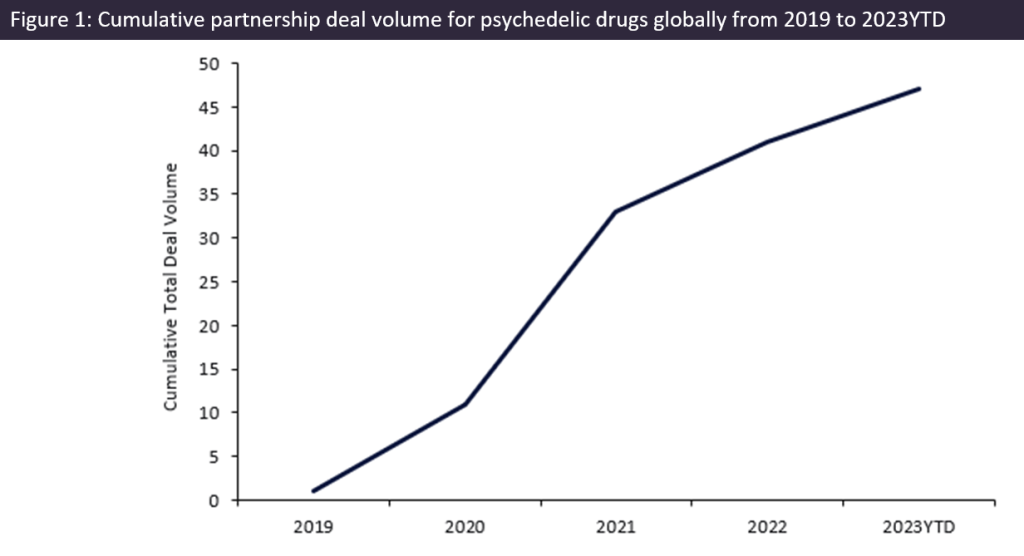
Partnership deals involving psychedelic drugs saw a 500% growth in total deal volume from 2019 to 2023 year-to-date (YTD), with more than 40 partnerships for psychedelic drugs since 2019, according to GlobalData’s Pharma Intelligence Center Deals Database.
Psychedelic drugs such as those derived from psilocybin, lysergic acid diethylamide (LSD), and midomafetamine (MDMA), demonstrated promise in depression, anxiety disorder, and substance abuse. The US Drug Enforcement Administration (DEA) currently classifies psychedelic drugs as a Schedule I-controlled substance under the Controlled Substances Act, defined as drugs that offer no medical value and hold high potential for abuse, which has made the R&D of psychedelic drugs as potential therapeutics challenging. However, regulatory bodies are starting to recognise the clinical applicability of psychedelic drugs, with the FDA and EMA issuing their first guideline documentation this year to support clinical trials and development of psychedelic drugs. In turn, the increase in partnership deals involving biopharmaceutical companies and research institutions could accelerate innovative psychedelic drug development in the future.
The US had the highest number of private biotech companies with 63 involved in psychedelic drug development, closely followed by Canada and the UK with 29 and 15 private biotechs, respectively. Health Canada was the first regulatory authority to approve a clinical trial for psilocybin-assisted therapy, in August 2020. Canada’s Special Access Program amendments in January 2022 enable healthcare practitioners to request psychedelic drug access for patients.
In September 2023, the biotech company Clearmind Medicine, headquartered in Canada, entered a co-development partnership deal with Johns Hopkins University School of Medicine to conduct a Phase I/II trial of its serotonin receptor 1A agonist, CMND-100, for the treatment of alcohol use disorder.
In June 2023, Clearmind Medicine also entered a co-development partnership deal with Hebrew University of Jerusalem and Israel-based company SciSparc to evaluate Clearmind Medicine’s CMND-100 in combination with SciSparc’s dual cannabinoid receptor 1/2 agonist palmidrol for the treatment of obesity and metabolic syndrome. This research agreement was a part of Clearmind Medicine’s ongoing collaboration with SciSparc, forged in March 2022, focusing on the development of innovative psychedelic drugs targeting mental health conditions.
Meanwhile, the Australia-based biotech company Psylo signed a co-development partnership deal with Daiichi Sankyo to support the advancement of its discovery and preclinical stage pipeline of non-hallucinogenic psychedelic drugs indicated for the treatment of chronic mental illnesses, including serotonin receptor 2A agonists PSYLO-3001, PSYLO-3002, PSYLO-5001, and PSYLO-1001.
The increase in partnership deals involving innovator psychedelic drugs over the last five years could see further clinical trials with the potential to gain regulatory approval in indications such as depression, ADHD, eating disorders, and addiction. However, biopharmaceutical companies developing psychedelic therapeutics require careful consideration of clinical trial design for psychedelic drugs and must demonstrate a favourable risk-to-benefit ratio to succeed in bringing their drugs to market.