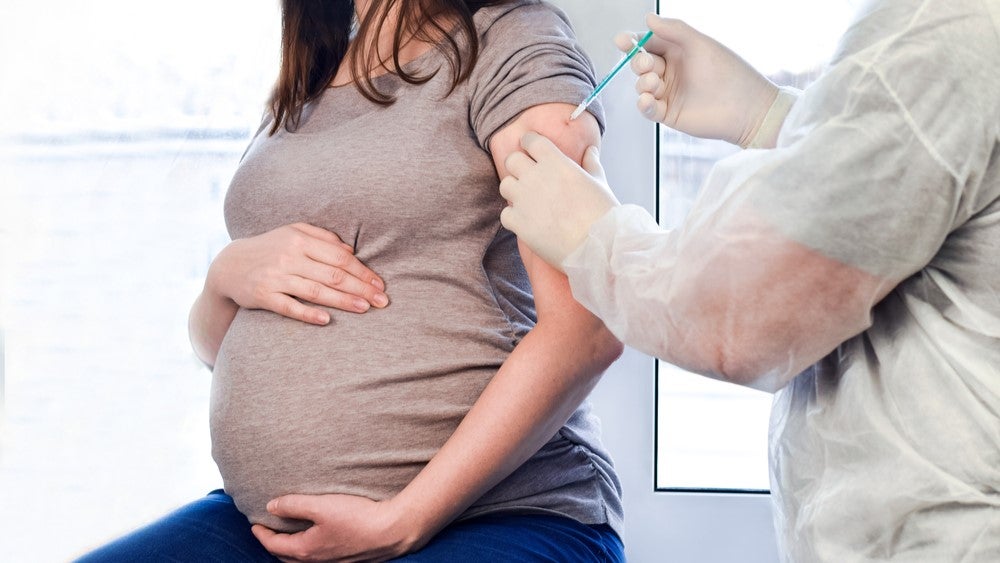
MinervaX has raised €54m ($57.4m) in upsized financing to advance the development of its group B streptococcus (GBS) maternal vaccine.
The proceeds raised as part of the financing round are expected to fund the Phase III trials of the GBS vaccine in 2024.
Infections caused by GBS bacteria are one of the most common diseases in newborns. These bacteria commonly live in gastrointestinal and genital tracts in adults, usually without causing any infections.
The Centers for Disease Control and Prevention (CDC) estimates that 1 in 4 have GBS bacteria in their body and it is commonly passed from mother to baby during delivery. GBS is associated with a high risk of morbidity in babies.
MinervaX’s GBS vaccine is a recombinant protein vaccine. In September 2022, it was awarded PRIority Medicines (PRIME) status by the European Medicines Agency due to its potential to prevent life-threatening infections in newborn babies.
The GBS vaccine is currently being evaluated in two Phase II trials (NCT04596878 and NCT05154578), including a subset of mothers who have a compromised immune system due to human immunodeficiency viral (HIV) infection.
The investors that took part in the funding round included EQT Life Sciences, OrbiMed, Novo Holding and Sanofi Ventures. Vincent Brichard of EQT Life Sciences and Tal Zaks of OrbiMed will also join MinervaX’s board of directors.
In December 2022, MinervaX raised €72m in funding for the GBS vaccine development programme. Following the current round of financing, MinervaX noted that its current financial reserves were more than €125m, based on an 11 October press release.
In April, the Danish company also initiated a dose-determining Phase I trial (NCT05782179) for the GBS vaccine in older adults aged 55–75 years.
Another GBS vaccine currently in development is Pfizer’s hexavalent capsular polysaccharide (CPS) conjugate vaccine. Placebo-controlled Phase II trial (NCT03765073) results were published in the New England Journal of Medicine in July.