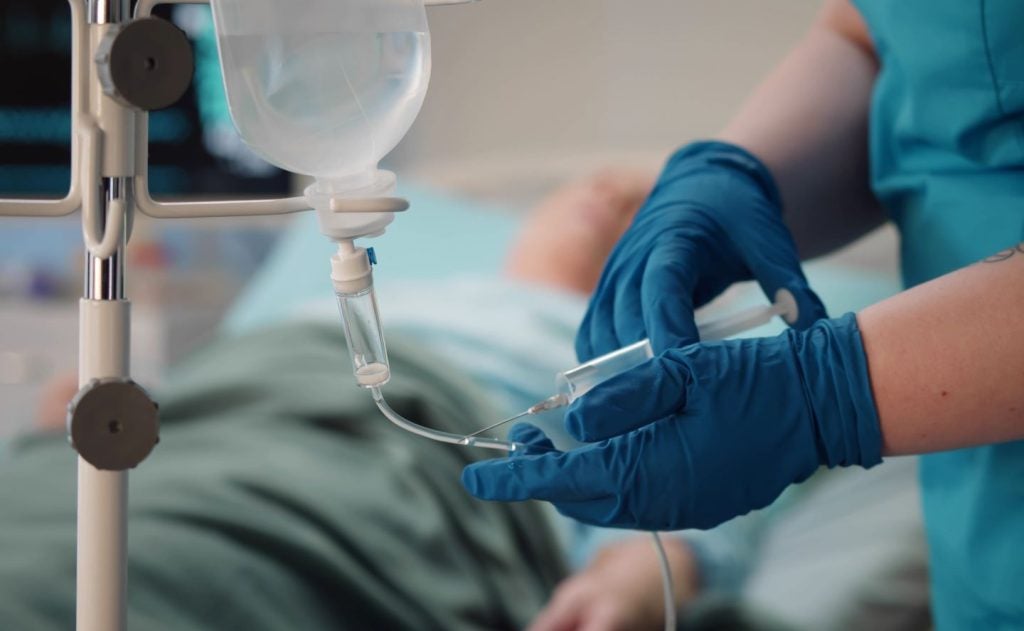
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved Napp Pharmaceuticals' new antifungal treatment for invasive candidiasis, Rezzayo (rezafungin).
The fungal infection is caused by the yeast candida, which can affect areas of the body such as the blood and vital organs.
Rezzayo is given as a weekly intravenous drip for at least 14 days until candida is no longer present in the bloodstream.
Rezafungin is the active ingredient of the treatment and works by inhibiting an enzyme essential for fungal cell wall synthesis, rendering the fungal cells fragile and halting their growth.
The approval of Rezzayo is based on the results of a Phase III randomised, double-blind and controlled clinical trial.
This study involved 187 invasive candidiasis patients who were randomly given rezafungin or another antifungal medication, caspofungin, for two to four weeks.
At the 14-day mark, 55 subjects treated with rezafungin were cured, versus 57 in the caspofungin arm.
By the 30th day, 22 subjects on rezafungin had died from various causes, in contrast to 20 on caspofungin.
Hypokalaemia, diarrhoea and fever were the most common side effects of Rezzayo.
MHRA healthcare quality and access interim executive director Julian Beach said: “Keeping patients safe and enabling their access to high quality, safe and effective medical products are key priorities for us.
“We’re assured that the appropriate regulatory standards for the approval of this medicine have been met. As with all products, we will keep its safety under close review.”