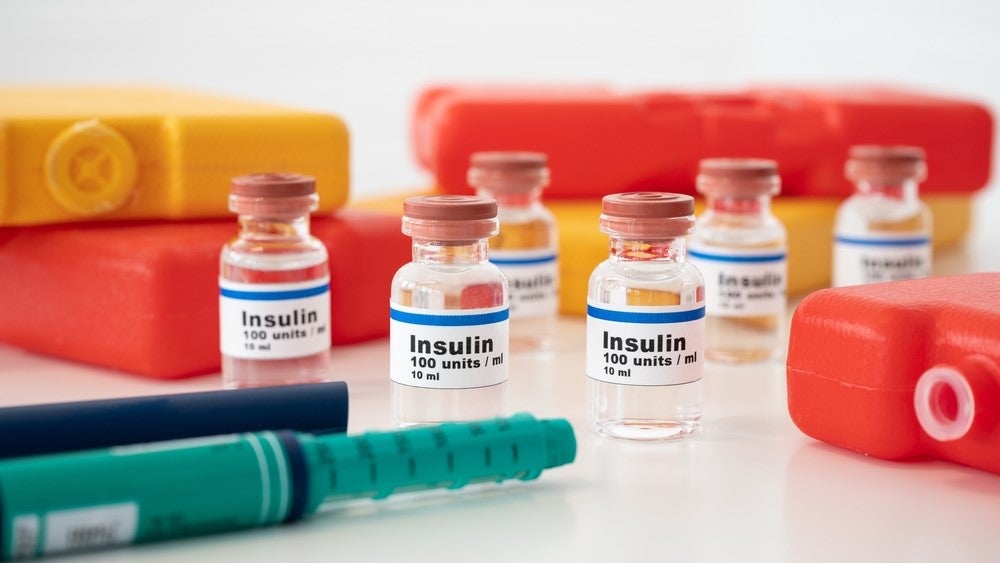
Meitheal Pharmaceuticals has signed an exclusive licensing agreement with the China-based Tonghua Dongbao Pharmaceutical to commercialise three insulin biosimilars in the US.
The insulin biosimilars include two rapid-acting insulins, namely insulin lispro and insulin aspart, and the long-acting insulin glargine. The rights were acquired by Meitheal’s parent company Nanjing King-Friend Biochemical Pharmaceutical.
Eli Lilly reported global sales of $440.4m for the branded version of insulin lispro Humalog in n Q2 2023, while Sanofi’s Lantus (insulin glargine) had global sales of $353m in the same period, as per the company’s Q2 financials.
Meitheal will have exclusive US marketing rights after the insulin biosimilars are approved by the US Food and Drug Administration (FDA), which is expected in 2026.
As part of the agreement, both Tonghua Dongbao and Nanjing King-Friend Biochemical would be responsible for the development and supply of all three insulin biosimilars. All three companies will also share the royalties from the US sales.
Although insulin suppliers such as Novo Nordisk, Eli Lilly, and Sanofi have reduced insulin prices in recent years, the cost of insulin remains quite high in the US. The list price for a 5-pack of Humalog 15mL is $530.40, as per Eli Lilly. The availability of biosimilars can help reduce the cost of insulin therapies, as has been seen in other therapy areas.
Currently available insulin biosimilars include, Biocon and Viatris’ Semglee—a Lantus biosimilar—which was the first insulin biosimilar approved by the FDA in 2021. Biocon also has a Novolog (insulin aspart) biosimilar, Kixelle, in its product catalogue. Kixelle has been approved in Europe.
Meitheal has a number of generic drugs as part of its marketed portfolio, including several cancer drugs such as azacitidine, a generic of Bristol Myers Squibb’s Vidaza. Although Vidaza was first indicated for treatment of myelodysplastic syndromes (MDS) it is currently under investigation for treating relapsed acute myeloid leukaemia (AML) and anaplastic large cell lymphoma (ALCL).