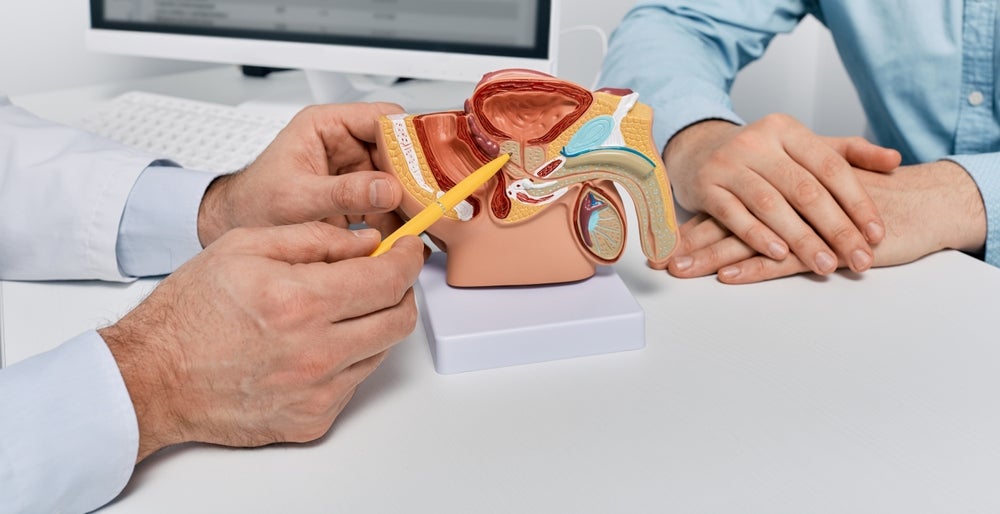
Lava Therapeutics announced that it has inked a clinical trial collaboration and supply agreement with MSD (Merck & Co) to evaluate its prostate cancer therapy LAVA-1207 as a combination treatment with MSD’s Keytruda (pembrolizumab).
The agreement will see MSD supplying Lava with Keytruda for the dose escalation and expansion portion of a Phase I/IIa trial (NCT05369000) for therapy refractory metastatic castration-resistant prostate cancer (mCRPC). The LAVA-1207/Keytruda treatment arm is expected to begin in H1 2024. The Phase I/IIa study will also continue enrolment for its monotherapy arm alongside an IL-2 combination parallel cohort.
The Dutch company previously announced at the 2023 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) that initial data demonstrated that drug had a favorable safety profile as well as preliminary signs of anti-tumour activity.
LAVA-1207 is a bispecific monoclonal antibody that acts by engaging prostate-specific membrane antigen (PSMA) to activate Vγ9Vδ2 (Vgamma9 Vdelta2) T cells that selectively target PSMA-positive tumour cells. The intravenously administered therapy is being developed using Lava’s proprietary Gammabody platform.
Since announcing the collaboration, Lava’s stock has jumped 49.4%.
Lava also has partnerships with Seagen and Janssen. The immuno-oncology company has a license agreement with Seagen for SGN-EGFRd2 (LAVA-1223), a drug candidate for advanced EGFR-positive solid tumours being evaluated by Seagen in a Phase I study (NCT05983133). As per an August 2023 financial update, the therapy’s investigational new drug (IND) application was cleared by the US Food and Drug Administration (FDA). In March 2023, Pfizer signed an agreement to acquire Seagen for $43bn. The company is collaborating with Janssen for an undisclosed project in preclinical development. The partnership triggered a milestone payment from Janssen in July 2023.