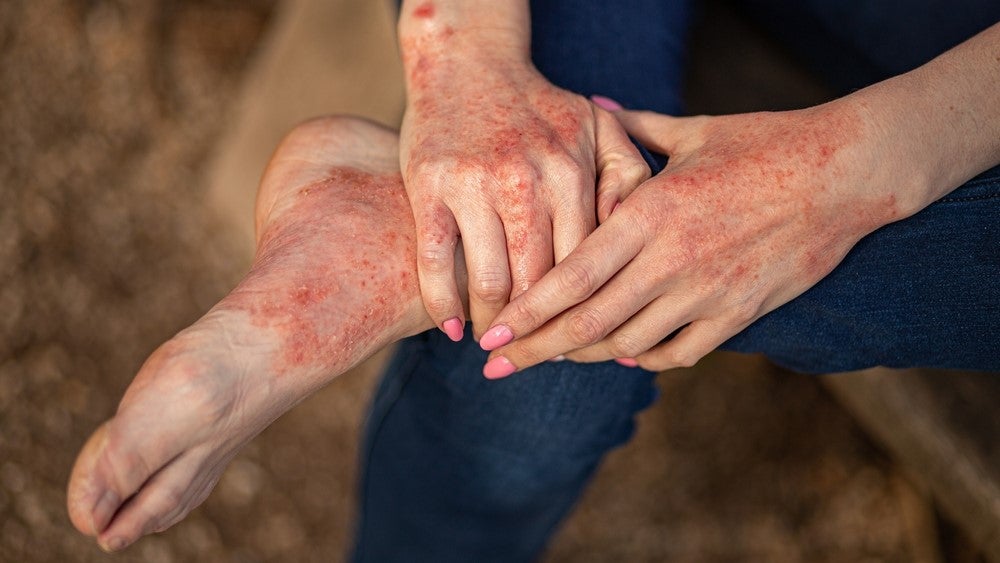
Kymera Therapeutics has dosed the first patient in the Phase II ADVANTA trial evaluating KT-474 in patients with atopic dermatitis (AD).
The company received a $15m milestone payment from Sanofi as part of a collaboration ahead of the Phase II trial (NCT06058156) initiation. In 2020, Sanofi signed a global licensing and development agreement with Kymera, as per which the latter received $150m in upfront payments and was entitled to receive over $2bn in milestone payments.
Kymera received $40m from Sanofi in October, following the start of a Phase II trial (NCT06028230) for KT-474 in patients with hidradenitis suppurativa (HS), a chronic inflammatory skin ailment. The company expects both the AD and HS trials to be completed in Q1 2025, as per a 7 December press release.
KT-474 is an interleukin-1 receptor-associated kinase 4 (IRAK-4) protein degrader. It is being developed as a treatment for interleukin 1R (IL-1R) / toll-like receptor (TLR)-driven complex inflammatory diseases.
The AD market in the 68 major markets is forecasted to grow from being worth $11.4bn in 2020 to approximately $24.4bn in 2030, as per a GlobalData market analysis report.
GlobalData is the parent company of Pharmaceutical Technology.
Phase I HS and AD data
The results from the Phase I trial (NCT04772885) of KT-474 in a healthy volunteer cohort showed degradation of IRAK4 in the participant's blood. A mean reduction of over 93% of IRAK4 was seen at 600mg–1,600mg and a reduction of over 95% was observed after 14 daily doses at 50mg–200mg.
The Phase I trial also enrolled 21 patients with either moderate to severe HS or moderate to severe AD in an open-label cohort. A reduction of disease-relevant inflammatory biomarkers was demonstrated in the blood and skin of patients with HS and patients with AD and was associated with improvement in skin lesions and symptoms. The results were also published in the Nature Journal in November.
Other protein degraders in Kymera’s pipeline include KT-333 for the treatment of relapsed or refractory lymphomas, large granular lymphocytic leukaemia, and solid tumours and KT-413 for the relapsed and/or refractory B-cell non-Hodgkin’s lymphomas. The positive data from the ongoing Phase I trials for both therapies was reported in June.