Artificial intelligence (AI) is widely considered to be the technology that will offer the most benefits for clinical trials. In GlobalData’s State of the Biopharmaceutical Industry annual survey to pharma professionals, AI came out on top for tech with the greatest sector impact in the consecutive years of 2020-2023. But which type of AI holds the most promise?
While AI’s potential applications are numerous, it is paramount that pharma companies looking at AI adopt a proactive, value-driven strategy rather than implementing tech for its own sake. Large language models (LLMs) such as ChatGPT have stunned the world with their capacity to quickly draw inputs from across the internet to generate quantities of text to any given prompt.
This capacity for boundless creativity is exciting but also potentially troublesome, with LLMs prone to generating unreliable responses. Supervised machine learning (ML) often proves to be a better way of leveraging the benefits of AI in the clinical research space while mitigating some of the drawbacks associated with LLMs, like transparency, cost, and consistency.
LLMs are not reliable generators of information
Capable of understanding and generating coherent human language text over long passages, LLMs can power translation models, content creation, and automated customer support bots. Their capacity for traditional natural language processing (NLP) also edges out traditional ML methods, facilitating tasks such as classification, entity extraction, and relationship extraction.
However, even at this preliminary stage, unavoidable issues are emerging with LLMs. Computing costs remain high, as do the risks, including inconsistencies in output and regulatory hurdles. Especially problematic are “hallucinations,” responses generated by AI that appear genuine and convincing but are actually incorrect.
Consider the below example, where ChatGPT was asked to provide the MedDRA code for “heart attack.” The response was impressively fluent, clear, and authoritative – and the code it returned was a real MedDRA code. The problem was, it wasn’t the code for heart attack.
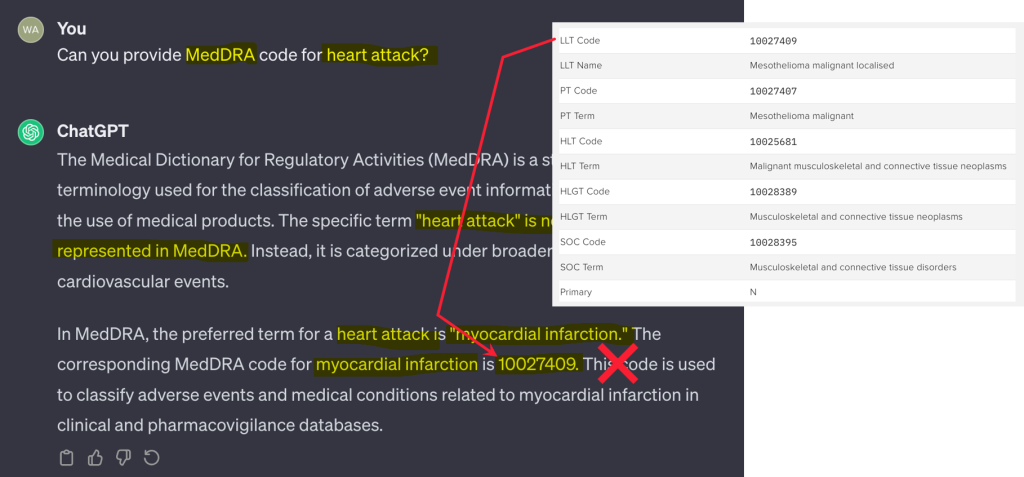
The full response gives the sense that ChatGPT has understood the question and possesses a deep knowledge of MedDRA. By using the correct language from MedDRA, for example the phrase “preferred term,” the LLM conveys an apparently deep knowledge of medical condition terminology. However, the medical code it has supplied in this case is actually the MedDRA code for mesothelioma, not myocardial infarction.
ChatGPT is not purposefully trying to mislead the user here. Rather, this hallucination is the result of the LLM choosing a highly probable next word at each position in the text as it generates the response. Anything that looks like a valid MedDRA code can be output, regardless of whether it is correct.
Therefore, careful consideration is required when constructing a solution around generative AI. Even adding a human validation process to AI-generated answers can be a wasted effort because the original generated text is so authoritative and persuasive.
However, supervised machine learning models have an inherent advantage as a result. Because they are assumed to be unreliable, they have no ability to ‘convince’ the user through a persuasive narrative. Rather, the user gains a level of familiarity and trustworthiness with the AI model.
The advantages of supervised machine learning
Given the concerns around data privacy and transparency, depending on the use case, LLMs may not be the ideal choice in certain clinical trials. For many use cases, supervised ML models run at a fraction of the cost of LLMs and are typically more effective, scalable, and maintainable.
Industry leaders such as Zelta by Merative are extending their philosophy of the right tool for the right job and putting customer value first to AI deployments in clinical studies. It’s easy to get sucked into the hype of LLMs, but investing in them – and steering your AI strategy exclusively toward LLMs – may be unnecessary when supervised ML models provide more efficient and concrete returns on investment. Zelta delivers clear, measurable value statements backed by pragmatic solutions, while avoiding buzzwords and overpromising on deliverables.
LLMs such as ChatGPT, Llama, and Gemini have made impressive advancements in language understanding and generation, offering valuable applications across various domains. However, Zelta has been finding more practical benefits in supervised machine learning to help sponsors and CROs meet their clinical trial objectives. Supervised ML allows greater control over the data, ensuring it meets the necessary standards for accuracy, compliance, and relevance. This approach reduces the risk of unpredictable outputs and enables them to fine-tune results for more reliable and actionable insights. While LLMs present exciting potential, the precision and oversight provided by supervised learning better serve their high impact needs at this stage.
For many clinical trials, supervised ML is ideal for meeting specific data requirements as opposed to other one-size-fits-all AI solutions. These more specific tasks also require less data, reducing the burden on managers training the models, and can be built into the workflow to create a strong feedback loop with human users.
While training data for supervised models can be difficult and expensive to acquire, long-term cost savings are significant, especially given the speed at which supervised ML models can run. When implemented the right way, they can make all the difference in transforming long-standing data challenges into new strategic advantages.
How Zelta is using AI to enhance clinical trial services
Industry leader Zelta has been integrating AI and advanced automation for more than a decade. The cloud-based eClinical platform makes managing clinical trials simple and easy, supported by AI for medical coding and CDASH annotation. This machine-learning software has helped save hundreds of hours of human labor and improved accuracy and efficiency in clinical trials.
With AI poised to continue having an outsized impact in clinical research studies, Zelta is committed to expanding its AI-enhanced features in the future – but only in accordance with tested use cases, rather than blindly following the crowd on the buzziest trends.
To learn more about Zelta’s approach of leveraging supervised machine learning for clinical studies, and their ten-year track record of leveraging AI and automation in the clinical space to deliver measurable results, download the report below.