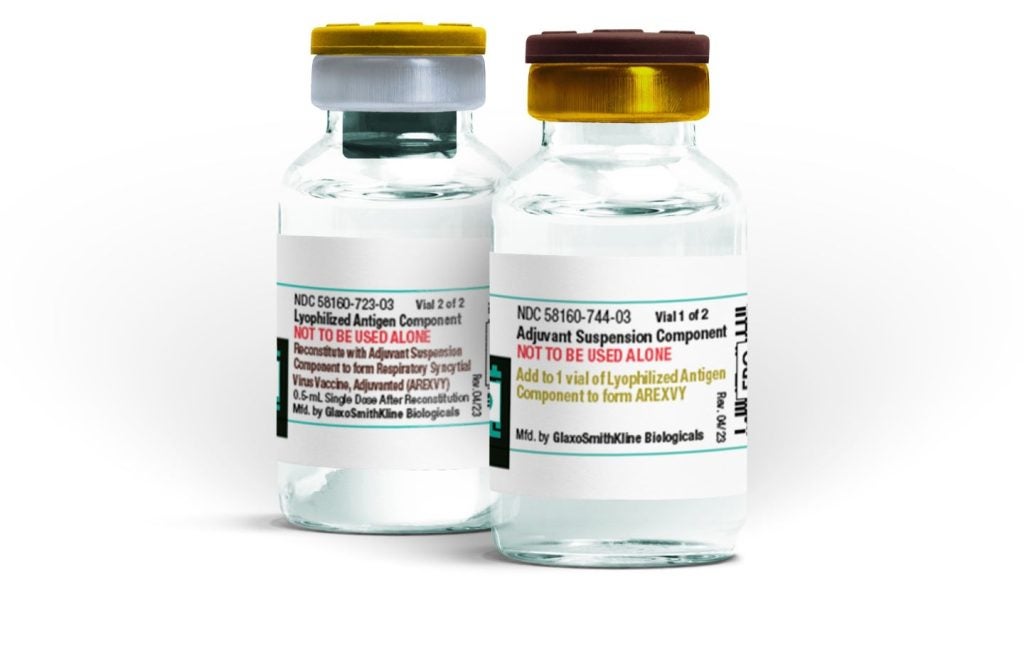
The US Food and Drug Administration (FDA) has expanded the age indication for GSK's Arexvy, making it the first vaccine against respiratory syncytial virus (RSV) available for adults aged 50 to 59 years at higher risk of lower respiratory tract disease (LRTD).
The approval extends the vaccine's previously authorised use for individuals aged 60 years and above.
The FDA decision is underpinned by the positive results from a Phase III clinical trial, which assessed the immune response and safety of Arexvy in the newly approved age group.
The trial was a placebo-controlled, observer-blind, randomised study in multiple countries, aiming to demonstrate the non-inferior immune response of the vaccine in adults aged 50 to 59 years versus those aged 60 years and above.
Safety and reactogenicity findings from the trial aligned with the initial AReSVi-006 data, with pain at the injection site being the most commonly reported local adverse event.
GSK is submitting the data to other regulatory bodies to support further label expansions.
Efforts to broaden the vaccine's use are already underway, with GSK having submitted applications in Europe, Japan and other regions.
Additional trials are also being conducted to evaluate the vaccine's immunogenicity and safety in adults aged 18 to 49 at increased risk, and immunocompromised individuals aged 18 and above. Results are expected in the second half of 2024.
Arexvy contains the recombinant RSV glycoprotein F stabilised in the prefusion conformation (RSVPreF3) and is adjuvanted with the company's AS01E.
GSK chief scientific officer Tony Wood stated: “The approval reflects the importance of broadening the benefits of RSV immunisation to adults aged 50 to 59 who are at increased risk.
“For those with underlying medical conditions, RSV can have serious consequences, so we are proud to be the first to help protect them from RSV-LRTD.”
The latest development comes after the company acquired Elsie Biotechnologies for $50m to unlock the potential of oligonucleotide therapeutics.