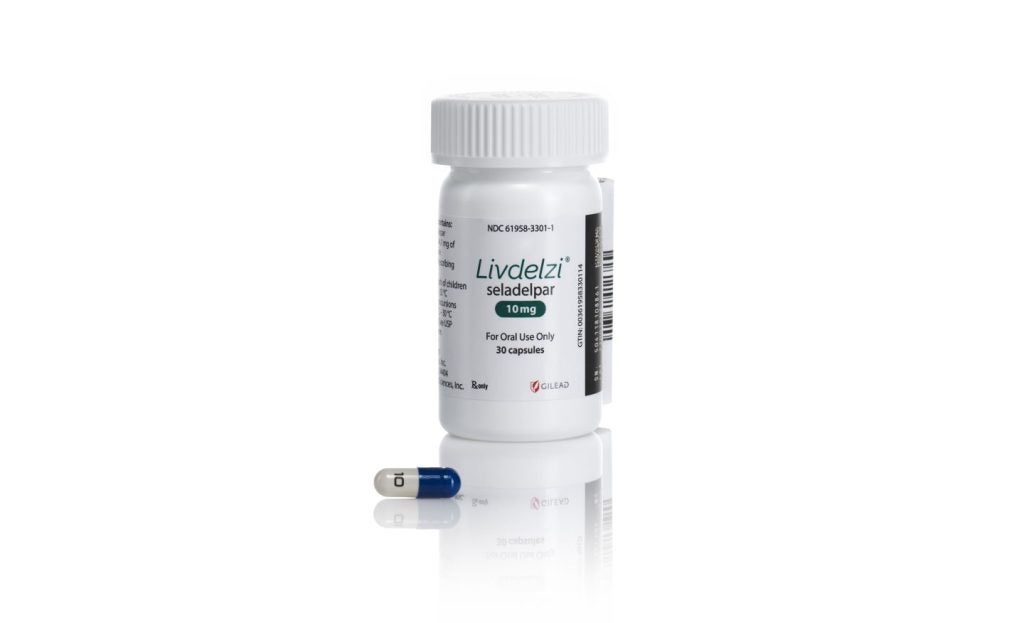
Gilead Sciences has formed a partnership with PANTHERx Rare to distribute LIVDELZI, a treatment for adults with primary biliary cholangitis (PBC).
The collaboration aims to provide patients with the selective peroxisome proliferator-activated receptor delta (PPARδ) agonist.
The US Food and Drug Administration (FDA) recently granted accelerated approval for LIVDELZI (seladelpar) to treat PBC in adults.
The approval is contingent upon the treatment demonstrating reduced alkaline phosphatase levels.
LIVDELZI is intended to be used in combination with ursodeoxycholic acid (UDCA) for PBC patients with an inadequate response to UDCA, or as a single agent for those who cannot tolerate it.
A rare, chronic liver disease, PBC predominantly affects middle-aged women, though men can also be diagnosed.
The disease is characterised by damage to the liver's small bile ducts, leading to cholestasis and the accumulation of toxic bile acids.
Symptoms such as fatigue and intense pruritus can severely affect patients' quality of life. Without effective treatment, PBC can cause irreversible liver damage and potentially lead to liver failure.
PANTHERx Rare specialises in the distribution of orphan drugs and providing essential support services. The company aims to improve the lives of those with rare and devastating conditions through access to medication breakthroughs and clinical excellence.
PANTHERx Rare Pharmacy executive chair Rob Snyder said: “People living with rare and devastating diseases deserve access to treatment options that work for them.
“The partnership with Gilead Sciences aims to positively impact key clinical outcomes for those suffering from Primary Biliary Cholangitis and we are pleased to be a part of that.”