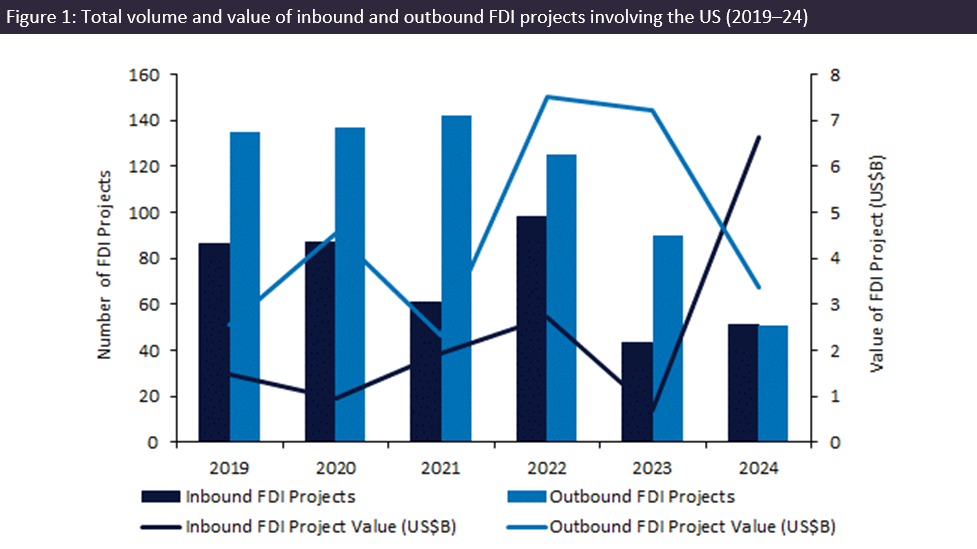
A 66-year-old man has become the fourth person in the world to receive a genetically edited pig kidney transplant, performed by surgeons at Massachusetts General Hospital (MGH) in the US.
Tim Andrews underwent the procedure at MGH in late January 2025. The transplant, part of a three-person compassionate use study, used a pig kidney with 69 gene edits provided by US-based biotech eGenesis. The company received the go-ahead from the US Food and Drug Administration (FDA) to start the study in December 2024, three months after securing $191m to fund its clinical programme.
Andrews – who had been on dialysis for more than two years due to end-stage kidney disease – faced significant challenges in receiving a human kidney transplant. His O-positive blood type meant he would likely have had to wait over five years for a suitable donor. The transplant has allowed him to stop dialysis for the first time since his diagnosis, and hospital officials report that his new kidney is functioning as expected.
“I believe that this is the start of something that is going to be fantastic. It’s going to be the option for people that don’t want to be on dialysis, they want to be able to be with their kids and their loved ones,” Andrews said in a video posted by MGH.
Xenotransplantation – whereby cells, tissues, or organs are transplanted from one species to another – is being hailed as a viable alternative to end waitlist mortality and alleviate transplantable organ shortages. According to the United Network for Organ Sharing (UNOS), more than 100,000 people in the US are waiting for organ transplants, with over 93,000 specifically waiting for a kidney.
Despite its promise, there are still some safety and ethical questions surrounding xenotransplantation. This is MGH’s second genetically edited pig kidney transplant in a living patient. The hospital performed the first such procedure in March 2024 to a 62-year-old man named Rick Slayman. He died two months later due to “unexpected, sudden cardiac causes,” though MGH stated there was no indication that his death was related to the transplant.
In addition to the two transplants carried out at MGH, NYU Langone Health has also conducted two genetically edited pig kidney transplants. The first was performed on Towana Looney, a US-based woman who – as of last month – became the longest-living recipient of a pig kidney. The second transplant was on 54-year-old Lisa Pisano, who received her kidney in April 2024. However, the organ was removed after six weeks due to blood flow issues, and she died in July 2024.
The procedures have been conducted under the FDA’s expanded access programme, which allows for experimental treatments in patients with life-threatening conditions. However, xenotransplantation is still in its early stages. on 3 February, the regulator cleared the first clinical trial for gene-edited pig kidneys, which will be conducted by eGenesis’ competitor United Therapeutics. The trial will initially involve six patients with end-stage kidney disease, eventually expanding to 50 participants. The first transplant is expected to take place in mid-2025.