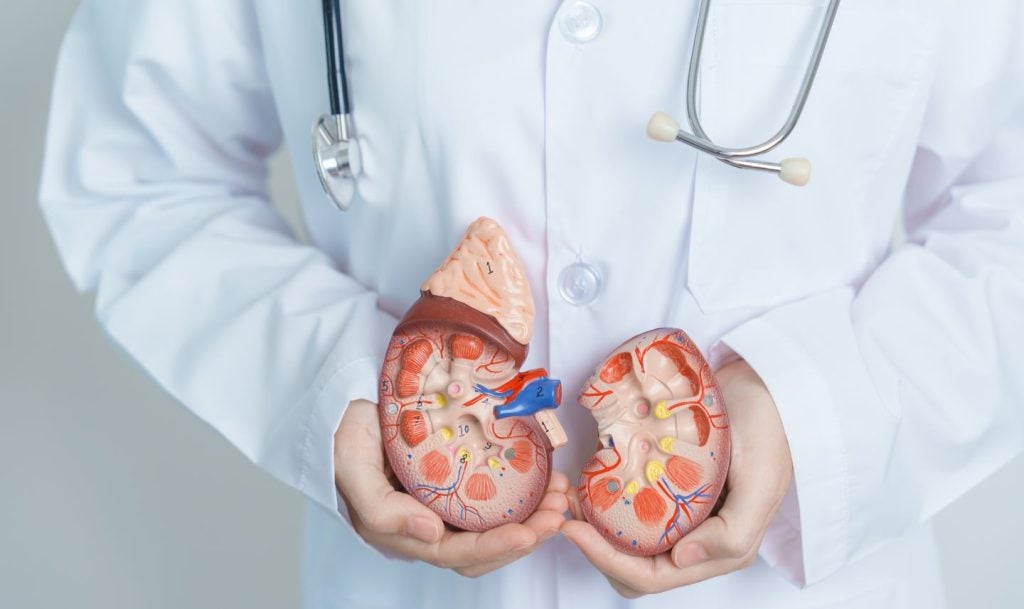
The US Food and Drug Administration (FDA) has accepted supplemental new drug application (sNDA) of Merck’s (MSD) Welireg for advanced renal cell carcinoma (RCC) and granted priority review.
Welireg is an oral inhibitor of hypoxia-inducible factor-2 alpha (HIF-2α). The treatment is intended for use in adult RCC patients subsequent to receiving immune checkpoint and anti-angiogenic therapies.
Merck filed the application based on findings from the Phase III LITESPARK-005 clinical trial that analysed Welireg against everolimus to treat advanced RCC patients.
According to the trial data, Welireg demonstrated improvement in progression-free survival (PFS) versus everolimus.
Furthermore, a statistically significant improvement in objective response rate (ORR) was observed.
The FDA is due to provide a decision on the approval of the treatment by 17 January 2024.
Merck Research Laboratories global clinical development late-stage oncology senior vice-president and head Dr Marjorie Green said: “Patients with advanced RCC whose cancer progresses following immune checkpoint and anti-angiogenic therapies face a poorer prognosis, and for those patients, there is a crucial unmet need for new options with an alternative mechanism of action.
“The FDA’s priority review designation of this application reinforces the urgency to provide new options to previously treated patients with advanced RCC, and we are committed to working closely with the FDA to bring Welireg to these patients as quickly as possible.”
Welireg is already approved in the US to treat von Hippel-Lindau disease in adults.
The latest development comes after the European Commission granted expanded indication approval for MSD’s Ebola Zaire vaccine, Ervebo, for children aged one year or above.