Concerns have been raised over the suitability of current FDA-approved treatments for myelofibrosis, due to the negative associations with anaemia. In response, the FDA has significantly increased the number of awarded orphan drug designations (ODDs) for myelofibrosis-indicated drugs in recent years. Since 2014, a total of 11 ODDs have been granted, with eight of them awarded post-2020. The high level of ODD awarding indicates the heightened focus by the FDA to explore alternative mechanisms of action, to tackle this unmet need within myelofibrosis.
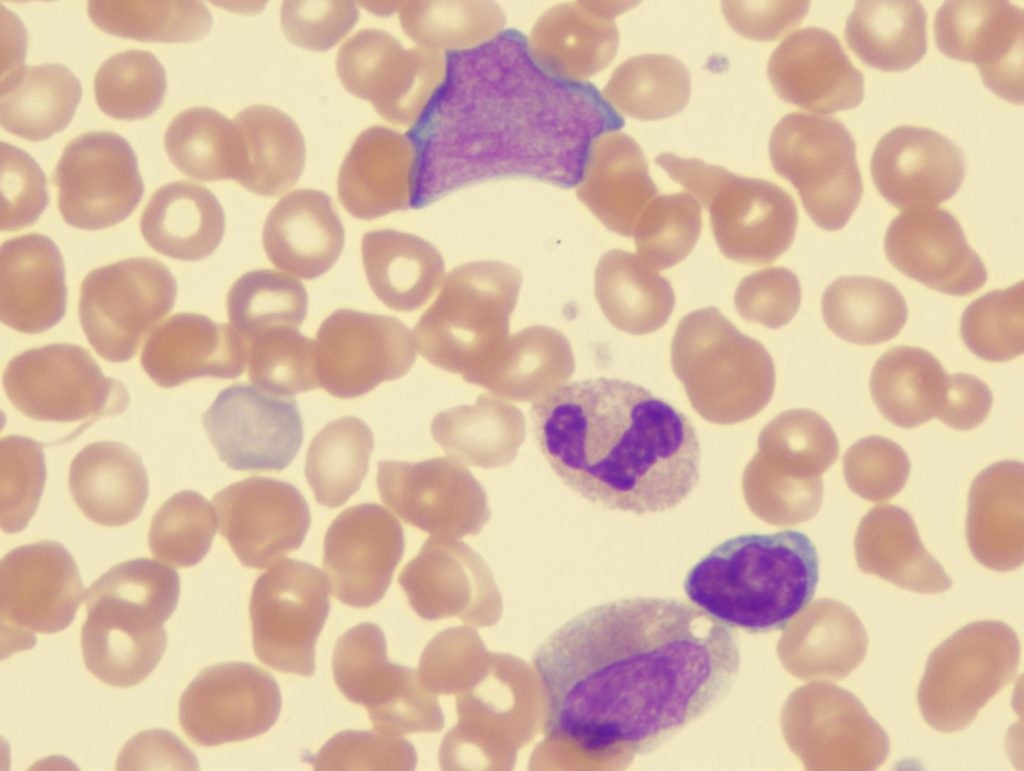
Myelofibrosis is an aggressive form of blood cancer that is characterized by the accumulation of scar tissue in the bone marrow. This buildup of scar tissue disrupts the body’s normal production of blood cells. This is a rare condition, with roughly 1.5 cases reported per 100,000 people annually in the US. Currently, there are four FDA-approved innovator drugs for this condition, all of which are Janus kinase (JAK) inhibitors. Although effective in treating myelofibrosis, this mechanism of action is well known for myelosuppression, which can induce and worsen symptoms of anaemia, reducing oxygen circulation throughout the body. As roughly half of the patients are already anaemic at diagnosis, there is a crucial need for approved myelofibrosis treatments that benefit this patient population.
The FDA has acted upon this need in recent years, via the increased awarding of ODDs (Figure 1). ODDs are a type of review designation that provides orphan designation status for drugs that have demonstrated promise for the treatment of rare diseases or conditions or are not expected to recover the costs of developing and marketing a treatment drug.
Figure 1: FDA orphan drug designations awarded to myelofibrosis drugs
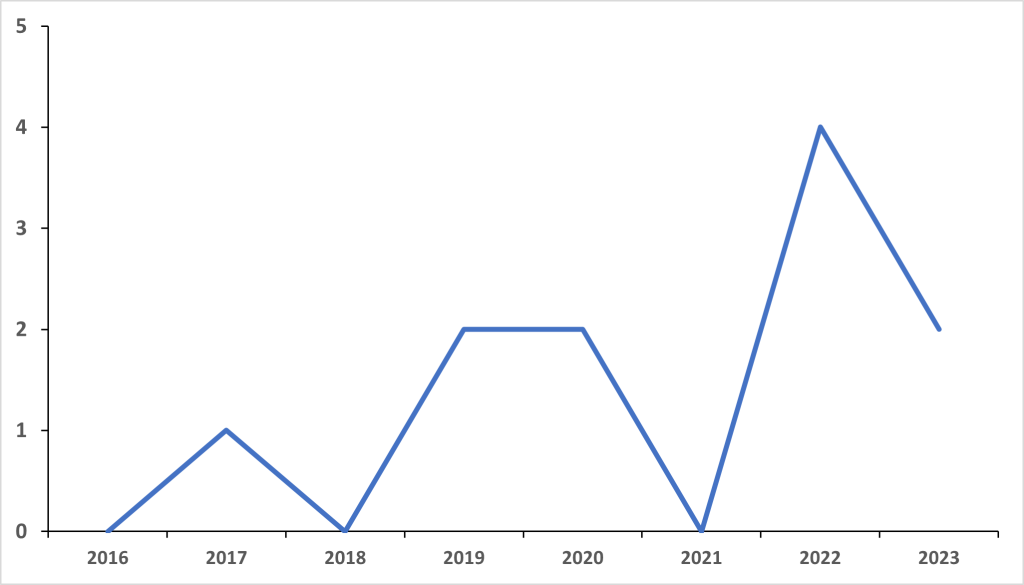
Since 2020, eight ODDs have been awarded by the FDA, which is more than double the number awarded over the preceding four years. 2022 saw a surge in the number of ODDs awarded by the FDA, with four being awarded, double the number of ODDs awarded in the previous peak years of 2019 and 2020, which both featured two.
Only two out of the 12 myelofibrosis drugs that received ODD by the FDA since 2014 act via JAK inhibition. This demonstrates the shift in regulatory focus from the FDA, away from this anemia-inducing mechanism of action. The remaining 10 drugs feature a variety of treatment mechanisms, with no two drugs sharing similar mechanisms of action. Merck & Co’s Reblozyl (luspatercept) acts via growth/differentiation factor (GFD) 11 and 8 inhibition. This recombinant fusion protein was awarded ODD by the FDA in January 2020 and is currently in Phase III for anemia-associated myelofibrosis.
The recent increase in the awarding of ODDs by the FDA highlights the enhanced effort to promote the development of novel myelofibrosis drugs that feature mechanisms of action that do not induce or worsen anaemia. This increase in awarded designations should lead to a greater number of approved treatments that offer greater benefits regarding this unmet area of myelofibrosis therapy.