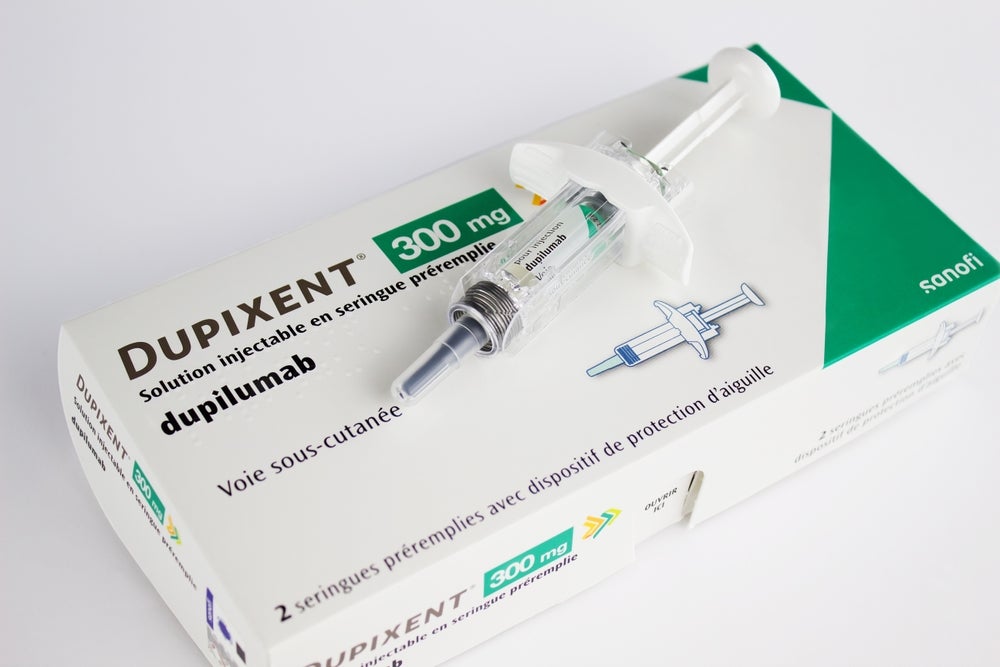
The US Food and Drug Administration (FDA) has expanded approval of Regeneron and Sanofi’s Dupixent (dupilumab) to children with eosinophilic esophagitis (EoE).
Patients aged 1–11 years and weighing at least 15kg with the oesophageal allergic inflammation will now have access to the drug, according to a 25 January press release.
EoE patients aged 12 years and older and weighing at least 40kg have had access to the blockbuster anti-inflammatory drug since May 2022, as per the initial FDA approval.
The FDA based its decision to expand treatment to more paediatric patients on data from the Phase III EoE KIDS trial. By week 16 of the higher Dupixent dose, 66% of children aged 1–11 years had disease remission compared with 3% of placebo. The trial showed results could be sustained for a year.
Sanofi said that the safety profile in younger children was generally similar to the one seen in older children and adults. The most common adverse events were injection site reactions, upper respiratory tract infections and joint pain, among others.
EoE is a chronic, progressive disease that damages the oesophagus and impairs its function. Around 21,000 children below 12 years of age are being treated for EoE in the US, Sanofi says.
Sanofi’s head of global development, immunology and inflammation, Naimish Patel, said: “Young children with eosinophilic esophagitis have significant unmet medical needs; despite existing treatment options, 40% of these children in the US under the age of 12 continue to experience symptoms of this disease.”
Dupixent is also FDA-approved for atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis. The drug, which has the same approval indications – including EoE – in Europe, generated global sales of $8.68bn in 2022. Global revenue for Dupixent was up 40% compared with 2021.
Dupixent is a monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways. It is jointly developed by Regeneron and Sanofi under a global collaboration agreement.
Sanofi said that the two companies are exploring further inflammatory and allergic diseases. Chronic pruritus – otherwise known as itchy skin – without a known cause and bullous pemphigoid – a blistering disorder – are in Phase III trials, according to Sanofi.
In November 2023, Sanofi and Regeneron reported positive results for the drug’s use in chronic obstructive pulmonary disease. The companies said they planned to submit data to the FDA by the end of 2023. Approval of the drug would make it the first biologic to treat the lung disease.