The US Food and Drug Administration (FDA) has approved Vertex Pharmaceuticals' CASGEVY (exagamglogene autotemcel [exa-cel]), a gene-edited cell therapy, to treat transfusion-dependent beta-thalassemia (TDT), a genetic disease.
It is indicated for use in TDT patients aged 12 years and above.
The therapy requires expertise in stem cell transplantation, prompting Vertex to collaborate with hospitals to create a network of authorised treatment centres (ATCs) across the US.
Nine ATCs in the US are prepared to administer CASGEVY to eligible patients with TDT and sickle cell disease (SCD).
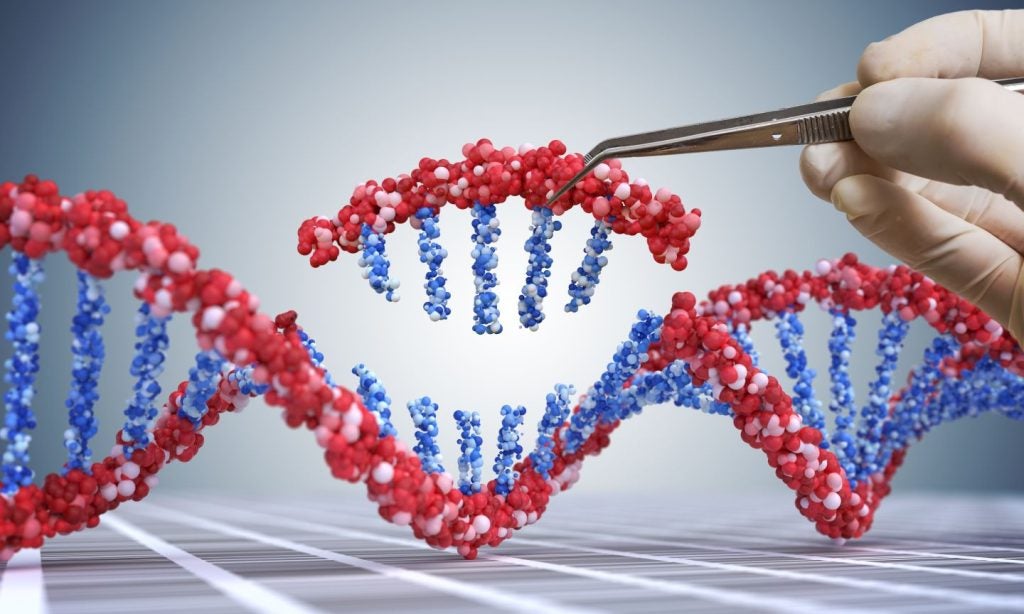
CASGEVY is a non-viral, ex vivo clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (CRISPR/Cas9) gene-edited cell therapy that modifies a patient’s own haematopoietic stem and progenitor cells.
These cells are edited at the BCL11A gene’s erythroid-specific enhancer region via a double-strand break.
This gene editing leads to the creation of increased foetal haemoglobin (HbF) levels in red blood cells.
HbF is the type of haemoglobin that carries oxygen in the foetus, which is replaced by adult haemoglobin after birth.
By increasing HbF levels, CASGEVY has demonstrated potential in reducing or removing vaso-occlusive crises in SCD and TDT patients who require blood transfusions.
CASGEVY has received approval for specific indications in several jurisdictions, providing a new treatment option for eligible patients.
Vertex Pharmaceuticals CEO and president Reshma Kewalramani stated: “On the heels of the historic FDA approval of CASGEVY for sickle cell disease, it is exciting to now secure approval for TDT well ahead of the PDUFA date.
“TDT patients deserve new, potentially curative treatment options, and we look forward to bringing CASGEVY to eligible patients who are waiting.”
This month, the company received approval for the gene-edited therapy for the same indication in Saudi Arabia.