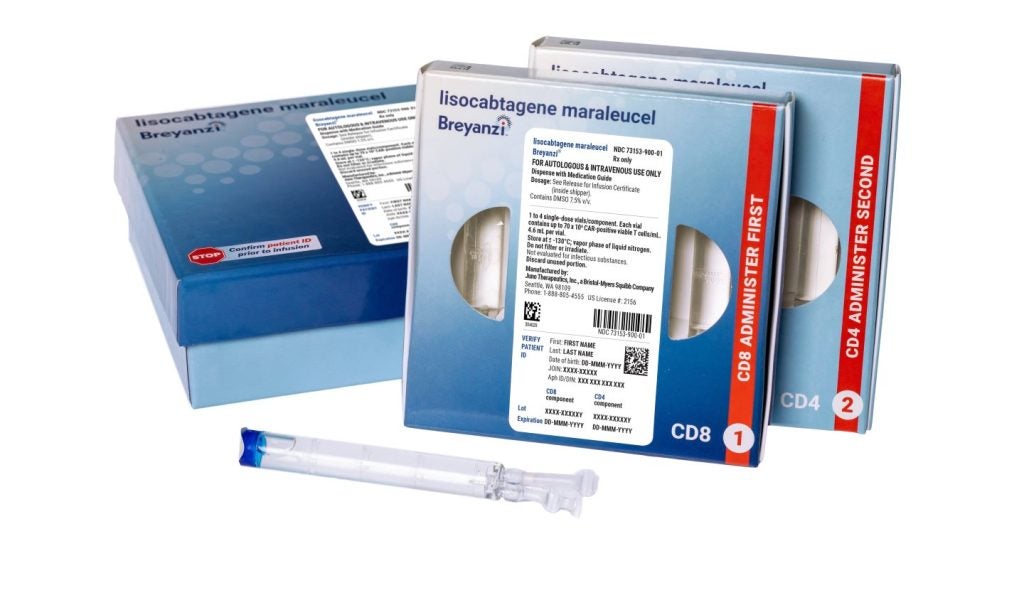
The US Food and Drug Administration (FDA) has granted accelerated approval for Bristol Myers Squibb’s Breyanzi (lisocabtagene maraleucel; liso-cel) for adult patients with relapsed or refractory follicular lymphoma (FL).
The CD19-directed chimeric antigen receptor (CAR) T cell therapy is indicated for patients who have undergone at least two previous systemic therapies.
Administered as a one-time infusion, Breyanzi delivers a dose of 90 to 110 × 10⁶ CAR-positive viable T cells to patients.
The approval is based on results from the open-label, global, multicentre, single-arm Phase II TRANSCEND FL clinical trial evaluating Breyanzi's efficacy and safety.
The primary outcome measure was the overall response rate while secondary measures included complete response rate, duration of response, progression-free survival and safety.
The trial demonstrated an overall response rate of 95.7% while the complete response rate was 73.4%.
Responses were reported to be quick, with a median time of one month, and durable. The median duration of response has not yet been reached.
At 12 and 18 months, the majority remained in response.
Breyanzi has shown a consistent safety profile throughout the trials.
Cytokine release syndrome (CRS) of any grade was reported in 53% of subjects with Grade >3 CRS seen in 4% of patients.
Bristol Myers Squibb cell therapy commercial head and senior vice-president Bryan Campbell stated: “Breyanzi is a cornerstone of our cell therapy portfolio, providing a differentiated profile across a wide array of B-cell malignancies.
“The approval of Breyanzi for relapsed or refractory FL provides an option with potential for lasting remission in a one-time infusion and a safety profile that allows for administration and monitoring in both the inpatient and outpatient setting in an increasing number of certified treatment centres in the US.”
Last month, the European Commission granted expanded approval for Bristol Myers Squibb‘s Reblozyl (luspatercept) as a first-line treatment for adults with transfusion-dependent anaemia associated with very low, low and intermediate-risk myelodysplastic syndromes.