The Danish artificial intelligence (AI) biotech Evaxion has announced plans to develop an mRNA vaccine against gonorrhea in partnership with Afrigen Biologics.
Gonorrhea antigens identified through Evaxion’s EDEN platform have shown strong protective effects in preclinical trials, as per the company. The partnership will investigate the function of these antigens when presented in the mRNA form. After the validation phase, the partners will discuss an agreement for clinical development and commercialisation, with the opportunity to involve additional collaborators. Cape Town, South Africa-based Afrigen Biologics is set up as a specialised centre to support mRNA vaccine development and technology transfer, will take charge of developing the mRNA vaccine in low and middle-income countries and African territories.
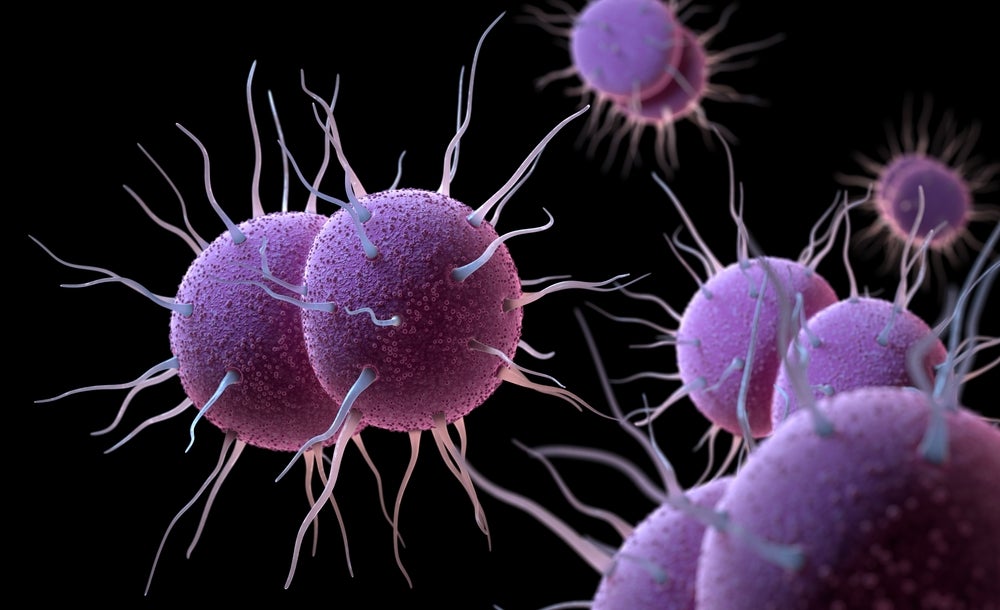
Gonorrhea is a sexually transmitted infection, caused by the bacteria Neisseria gonorrhoeae. It heightens the vulnerability to HIV, a prevalent health concern in many low and middle-income countries (LMICs). Birgitte Rønø, Evaxion’s Chief Scientific Officer said, “This partnership has the potential to address a serious unmet global medical need against the pathogen for which no vaccine currently exists.”
In June, GSK received a fast track designation for its own gonorrhea vaccine, NgG, which is in an ongoing Phase II study. Last year, the US National Institute of Allergy and Infectious Diseases (NIAID) awarded the Dutch biotech Intravacc $14.6m to develop an intranasal gonorrhea vaccine.
mRNA vaccines work by delivering synthetic messenger RNA (mRNA) into cells to prompt an immune response. They can be manufactured quicker and scaled up more efficiently in comparison to conventional vaccines. Pfizer and BioNTech's Covid-19 vaccine was the first mRNA product to achieve full FDA approval in the US in 2020. Since then, the industry has been moving towards mRNA vaccines for other infectious diseases.
According to GlobalData’s clinical trials database, 70% of all ongoing mRNA vaccine trials are in the infectious disease space. Additionally, 85% of planned mRNA vaccine trials are sponsored by industry in 2023, up from just 34% of mRNA trials initiated in 2021.
Moderna, which was highly successful in developing its own mRNA Covid-19 vaccine, currently has an mRNA vaccine called mRNA-1574 in Phase I clinical trials for the prevention of human immunodeficiency virus infections (HIV), among others. BioNTech is also running a Phase I (NCT05432583) 108-participant study for the BNT163 vaccine to prevent genital herpes lesions, which has an estimated primary completion date of Q2 2025.
GlobalData is the parent company of Pharmaceutical Technology.