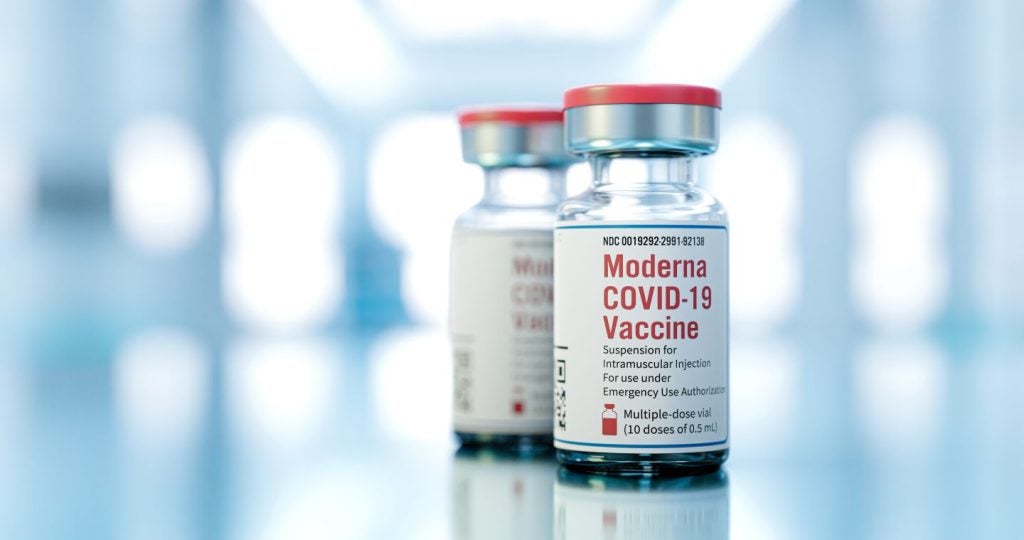
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended granting marketing authorisation for Moderna’s Spikevax, an updated Covid-19 vaccine targeting the SARS-CoV-2’s XBB.1.5 sublineage.
The vaccine is intended for active immunisation for the prevention of Covid-19 in those aged six months and above.
The latest development comes after the company filed a regulatory application seeking approval for the updated vaccine in July 2023.
Based on the positive opinion from the committee, the European Commission will adopt a decision on the vaccine approval for autumn/winter 2023.
Moderna has obtained clinical results for the monovalent XBB.1.5 vaccine candidate which offered an immune response against sublineages of XBB, XBB.1.5, XBB.1.16, and XBB.2.3.2, apart from BA.2.86, EG.5 and FL.1.5.1 variants.
Injection site pain was reported to be the most frequently solicited local adverse event for the updated vaccine.
Fatigue, myalgia, headache, chills and arthralgia were the most frequently observed adverse events in trials.
The safety profile of the updated vaccine was in line with those observed in prior formulations of Spikevax.
Moderna CEO Stéphane Bancel stated: “The CHMP’s positive recommendation for our updated Covid-19 vaccine is a key milestone, given we see increasing transmission of SARS-CoV-2 across Europe.
“Our updated Covid-19 vaccine generates a strong human immune response against circulating variants, including BA.2.86, EG.5, and FL.1.5.1, and will be a critical tool for protection.
“We are working with governments across Europe to include our updated Covid-19 vaccine in national vaccination programmes, to ensure a diversified portfolio that provides vaccine choice and access to single dose vial formats, which can limit waste.”
The company obtained approvals for the use of the updated vaccine in the US, Canada, Japan and Taiwan with regulatory submissions underway globally.