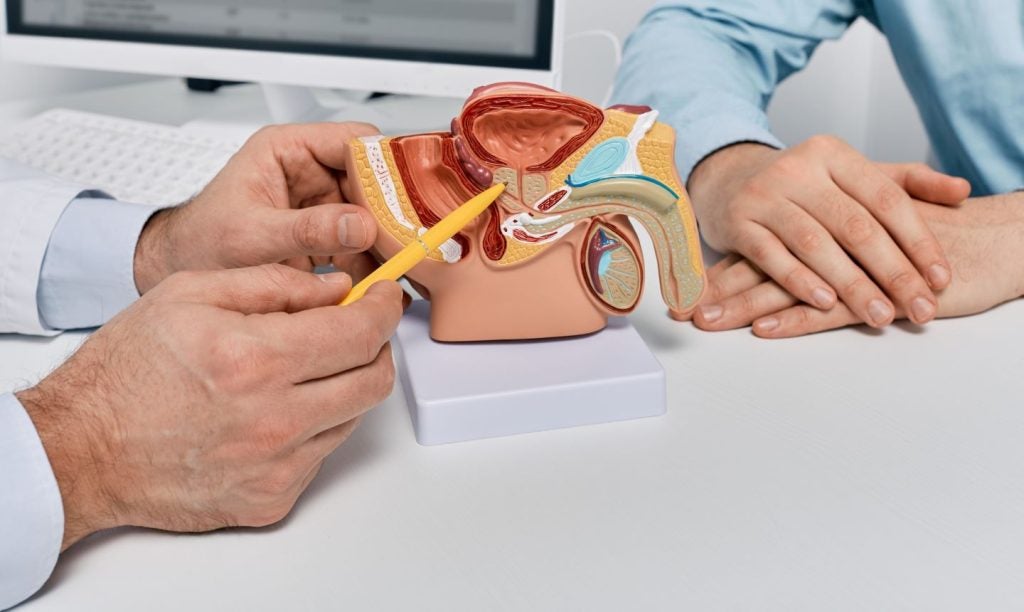
The European Commission (EC) has refused to grant marketing authorisation for Ipsen’s investigational therapy, palovarotene, intended to treat fibrodysplasia ossificans progressiva (FOP).
The EC’s decision comes after the Committee for Medicinal Products for Human Use (CHMP) provided a negative opinion in May 2023.
An oral therapy, palovarotene selectively acts on the retinoic-acid receptor gamma (RARγ).
It is the first-of-its-kind medicine to seek approval for FOP treatment.
Palovarotene has been analysed in a clinical programme, which included the Phase III MOVE clinical trial for FOP, for more than 15 years.
In the US, palovarotene obtained orphan drug and breakthrough therapy designations from the Food and Drug Administration to potentially treat FOP.
A decision on the approval by the US FDA is anticipated on 16 August 2023.
The company also noted that regulatory approvals will be sought in other nations.
Palovarotene obtained authorisation for usage in Canada and received provisional approval in the United Arab Emirates (UAE) as Sohonos (palovarotene capsules).
Ipsen research and development executive vice-president and head Howard Mayer stated: “We believe that our clinical data provide evidence supporting the effect of palovarotene on the reduction of new, abnormal bone formation, known as heterotopic ossification, which characterises the disease.
“We are therefore disappointed that the European Commission decided not to approve this treatment for patients with FOP in Europe.”
A chronic condition, FOP causes flare-ups which can lead to the formation of abnormal bones.