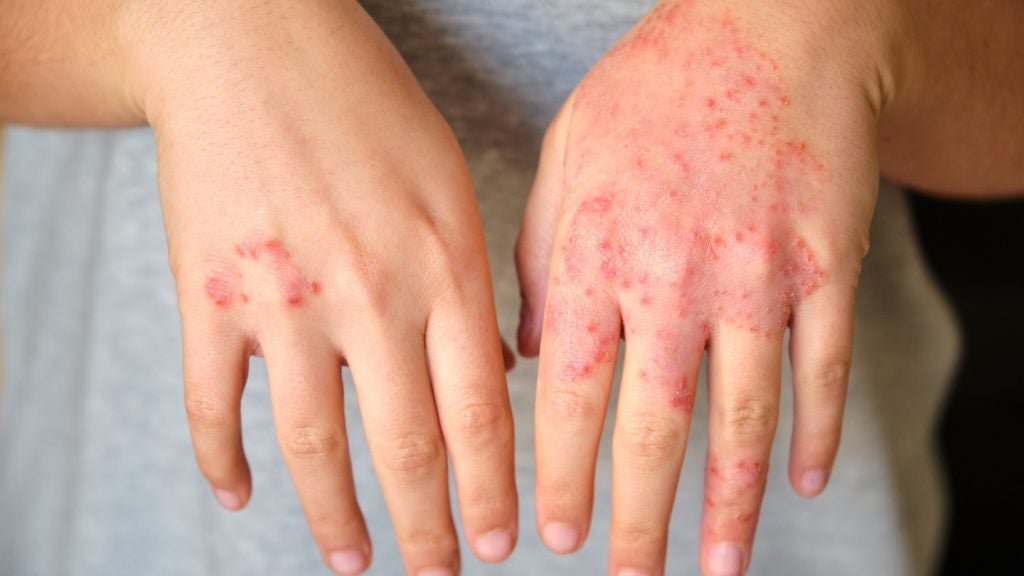
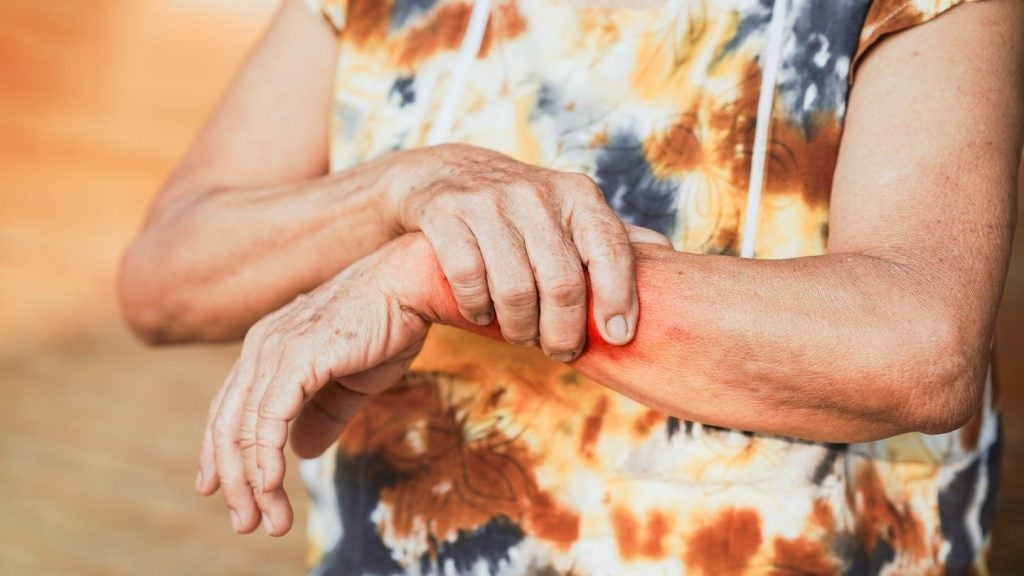
The European Commission (EC) has granted marketing authorisation to LEO Pharma’s Anzupgo (delgocitinib) cream for treating adults with moderate to severe chronic hand eczema (CHE).
The cream is indicated for use in CHE patients for whom topical corticosteroids are not suitable.
The approval extends to all member states of the European Union, Norway, Iceland and Liechtenstein.
The authorisation for Anzupgo cream is supported by data from the Phase III DELTA 1 and DELTA 2 clinical trials, which assessed the safety and efficacy of the cream versus a vehicle.
Both trials met their primary and all secondary endpoints.
LEO Pharma CEO Christophe Bourdon stated: “We are dedicated to advancing the standard of care for those living with skin conditions, and this long-awaited milestone for Anzupgo demonstrates our purpose in practice.
“This approval provides a new treatment option for patients, and we look forward to coordinating the next steps required to provide Anzupgo to those patients who need it.”
The US Food and Drug Administration (FDA) has also accepted the company’s new drug application seeking approval for delgocitinib cream.
A topical pan-Janus kinase (JAK) inhibitor, Anzupgo works by addressing the JAK-STAT signalling pathway implicated in CHE's pathogenesis.
LEO Pharma holds the sole rights for the development and commercialisation of delgocitinib cream for dermatological uses globally, except in Japan, since the signing of a licensing agreement with Japan Tobacco in 2014.
Japan Tobacco holds the rights to the asset in Japan.
LEO Pharma chief development officer Kreesten Meldgaard Madsen stated: “This approval is the culmination of years of focus and effort, driven every day by the goal to support this patient population.
“The new treatment option aims to support the management of this debilitating condition, given the limited treatment options for CHE currently approved across Europe.”
In June 2024, Leo Pharma received FDA approval for Adbry (tralokinumab-ldrm) single-dose autoinjector for atopic dermatitis.