Pipeline treatments across the seven major markets (7MM: France, Germany, Italy, Spain, the UK, the US and Japan) reflect a growing research trend prioritising disease-modifying therapies (DMTs) and non-motor symptom therapies in the treatment of Parkinson’s disease, according to new research.
The current therapeutic landscape for Parkinson’s is heavily genericised, exhibiting a notable lack of available neuroprotective treatments. However, Lorraine Palmer, pharmaceuticals analyst at GlobalData, says this is changing.
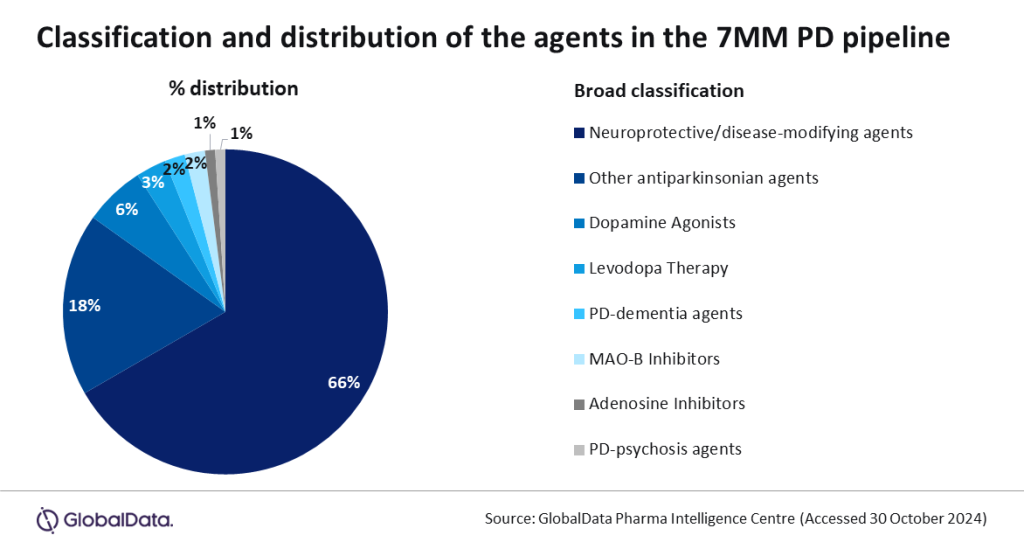
“There are a total of 93 products in Phase I-III development within the 7MM for the treatment of Parkinson’s,” Palmer said. “66% of these are prospective neuroprotective agents or DMTs. These investigational agents target the key mechanisms that have been implicated in the pathophysiology of Parkinson’s, such as alpha-synuclein aggregation and neuroinflammation, with the goal of slowing disease progression.”
GlobalData is Pharmaceutical Technology’s parent company.
DMTs look to be a particularly significant piece of the Parkinson’s puzzle, and key opinion leaders interviewed by GlobalData agreed on the need for further research focus, despite complications presented by the differing pathogenesis of Parkinson’s across patients.
One of the neuroprotective and DMT agents in late-stage development is Annovis Bio’s Posiphen (buntanetap tartrate). The drug is aimed at inhibiting alpha-synuclein, and it is currently in Phase III development in the US and five major European markets. Elsewhere, a Phase III trial in the US has been planned for BioVie’s Triolex (bezisterim), which is an inhibitor of inflammatory mediators and is thought to offer neuroprotective potential.
Palmer added: “26% of the pipeline DMTs in the pipeline target alpha-synuclein aggregation. This is a divisive mechanism of action, with some key opinion leaders holding high hopes and others expressing scepticism, highlighting the failure of Prothena and Roche’s prasinezumab to meet its primary endpoint in the Phase II PASADENA trial (NCT03100149) and safety concerns due to the role of alpha-synuclein in healthy functioning.”
Alongside DMTs, treating non-motor symptoms is also becoming a key research focus, highlighted by analysis which shows that 3% of the pipeline is specifically targeting Parkinson's disease dementia (experienced by up to 80% of people with Parkinson’s) and Parkinson’s disease psychosis (experienced to some degree by between 20% and 40% of people with Parkinson’s).
Research development appears to be addressing a broader spectrum of Parkinson’s challenges, and there is interest in antiparkinsonian agents, dopamine agonists and treatments for Parkinson’s disease related dementia. It suggests that the industry is looking to improve both motor and non-motor symptoms, addressing the broader spectrum of challenges faced by Parkinson’s patients.
Palmer concluded: “The distribution of the 93 pipeline agents in the 7MM underscores a strong emphasis on neuroprotective and disease-modifying treatments, demonstrating an industry-wide focus toward therapies that could potentially slow the progression of Parkinson’s.”









