Clinical-stage biotech Chemomab Therapeutics has announced the publication of a study highlighting that high serum CCL24 levels are linked to more severe forms of systemic sclerosis (SSc).
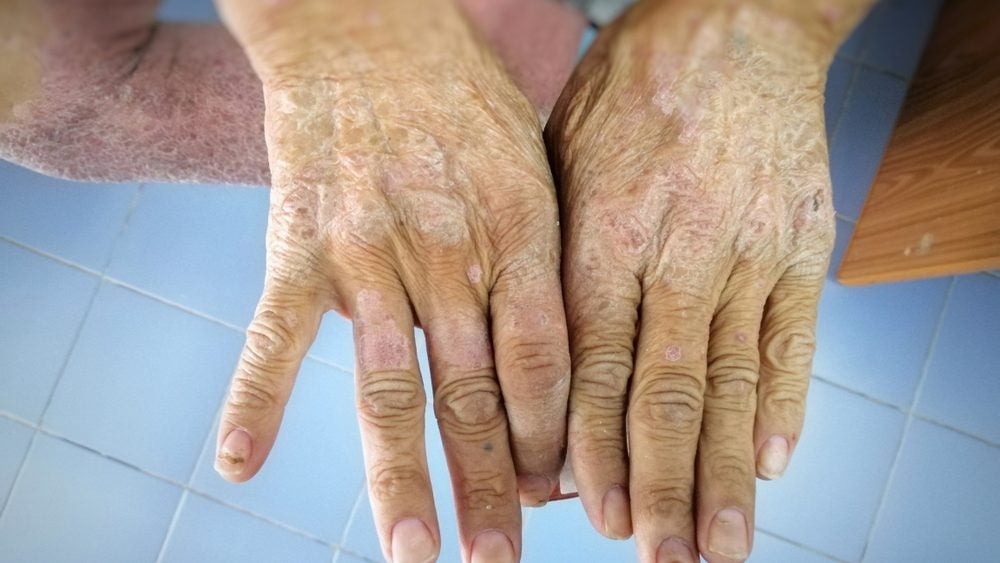
SSc is a chronic autoimmune disease characterised by excessive collagen production, leading to thickening and hardening of the skin and various internal organs. Affecting the skin, blood vessels, muscles, and internal organs, the condition causes symptoms like joint pain, skin tightening, and organ dysfunction.
The study, published in the Arthritis Care and Research journal and conducted at the University of Leeds in the UK, looked at 200 patients with SSc. It found that a quarter of them had high levels of CCL24 in their blood, even though they were receiving standard treatment. Higher CCL24 levels were linked to more severe forms of the condition, including worse skin problems and lung issues.
Scientists identified that high CCL24 levels were predictive of lung problems worsening over time, and patients with higher levels of the protein had a higher risk of dying from SSc within 10 years, which they said validated CCL24 as an important biomarker.
Chemomab is developing its own monoclonal antibody, CM-101, that neutralises CCL24. Standard treatments for SSc usually involve steroids, immunosuppressants known as conventional disease-modifying anti-rheumatic drugs (DMARDs), or biologic treatments. Genentech’s subcutaneous injection Actemra (tocilizumab) was the first biologic to receive FDA approval in March 2021 to treat the condition.
CM-101 is currently in a Phase II trial (NCT04595825) for primary sclerosing cholangitis (PSC), with data expected later on in 2024. In February 2023, Chemomab received clearance from the US Food and Drug Administration (FDA) to initiate the Phase II ABATE trial (NCT06210945) of CM-101. The company plans to start enrolment on that study following topline results from the PSC trial, Chemomab’s CEO Adi Mor said in the 18 April release announcing the study.
“These results also reinforce our belief, based on multiple preclinical and patient sample studies, that our novel CCL24-neutralizing antibody CM-101 has substantial potential as a treatment for SSc,” adds Mor.
CM-101 was being investigated in a clinical trial for non-alcoholic steatohepatitis (NASH), however Chemomab announced in June 2023 that it was parking the trial and putting its resources into PSC, despite initial promising results.