The US Food and Drug Administration (FDA) has granted Certa Therapeutics’ FT011 orphan drug designation for the treatment of scleroderma.
In line with the orphan drug status, the company is now eligible for tax credits for qualified clinical trials, exemptions for certain FDA fees, and a potential seven years of market exclusivity if FT011 is approved.
Certa recently conducted a Phase II study (NCT04647890) with FT011, an orally taken anti-fibrotic agent, in patients with scleroderma.
Full data from the trial will be presented at the American College of Rheumatology Annual Meeting 2023 being held on 10-15 November 2023, in California, US, based on a 23 October press release.
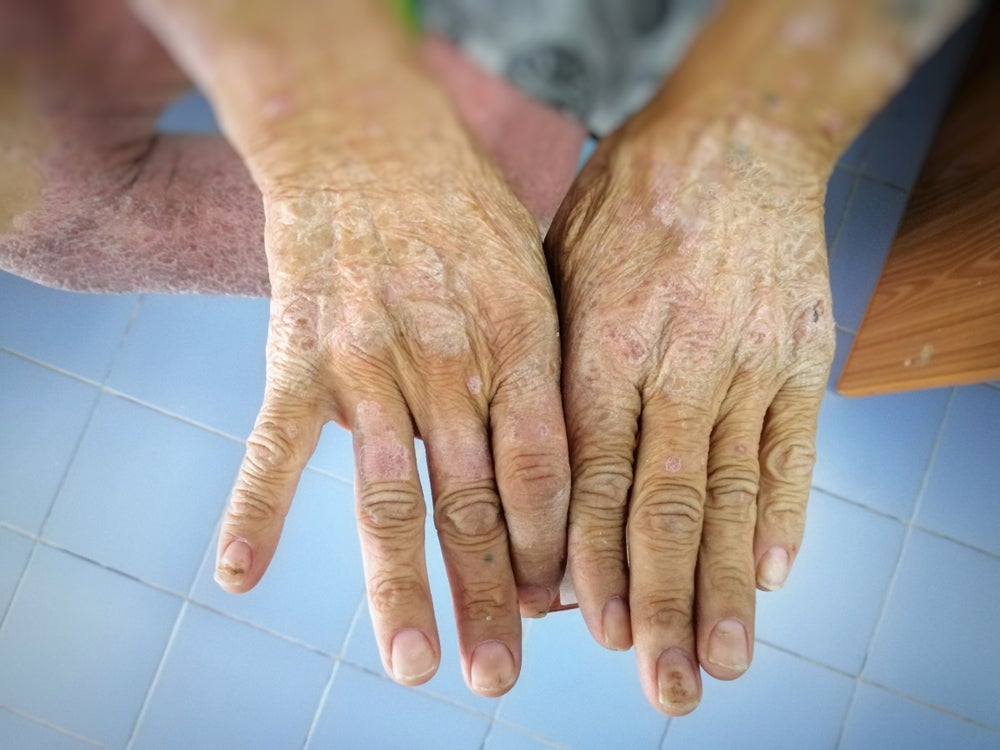
In February 2023, Certa announced topline results from the study and announced efficacy results from the trial. The use of 400mg of FT011 led to a clinically meaningful improvement in 60% of patients. Patients treated with the compound improved in measures such as the American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis (CRISS) score and skin thickness as measured by the modified Rodnan Skin ScoreCerta reported no serious adverse events.
As of February 2023, an open-label extension was ongoing and some of the patients opted to remain on FT011 treatment for a further nine months.
Also known as systemic sclerosis, scleroderma is an autoimmune disease characterised by inflammation and fibrosis of the skin and organs, including the heart, lungs and kidneys.
FT011 works by targeting a G protein-coupled receptor (GPCR) in the fibrosis signalling pathway. Certa states it has identified this GPCR is silent in healthy tissue but activated in certain diseases or following injury. As with many GPCRs, the protein has multiple downstream signalling effects. The company says that preventing the pathways that lead to fibrosis via inhibition of the receptor could prevent the disease in patients, potentially slowing the rate of disease progression.
Though there are no approved therapies treating the underlying cause of scleroderma, there are drugs approved in the US for treating associated complications from the disease. These include Roche’s Actemra (tocilizumab) for scleroderma-associated lung fibrosis and Boehringer Ingelheim’s Ofev/Vargatef nintedanib for scleroderma-associated interstitial lung disease.
GSK is also targeting scleroderma treatment with its B-cell inhibiting monoclonal antibody Benlysta (belimumab). Its candidate received an FDA orphan drug designation in February 2023.