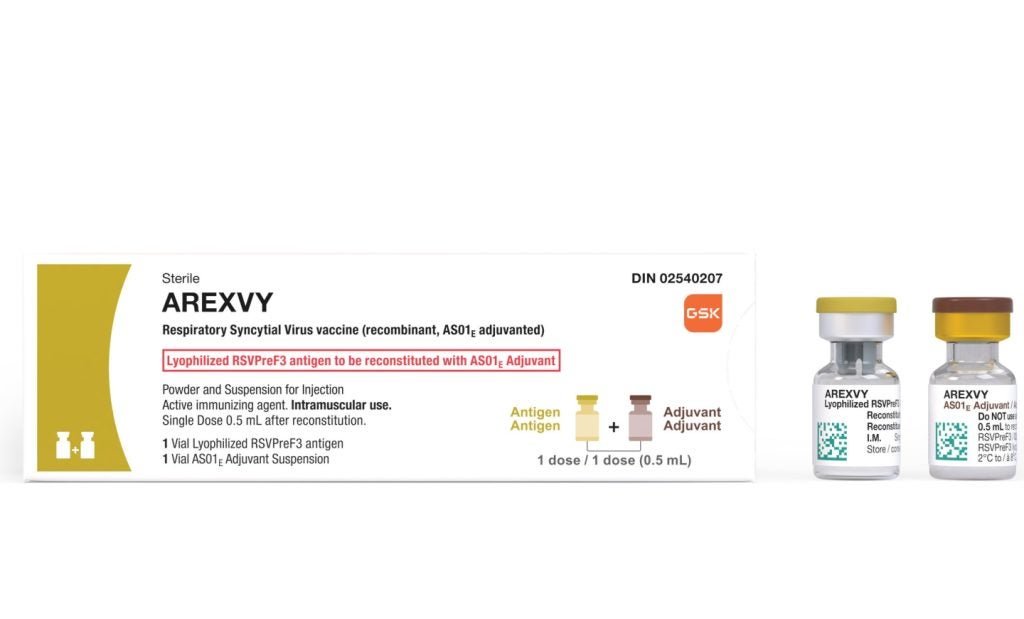
Health Canada has granted approval for GSK’s Arexvy vaccine to prevent respiratory syncytial virus (RSV)-related lower respiratory tract disease (LRTD) in people aged 60 years and above.
A recombinant, AS01E-adjuvanted RSV vaccine, Arexvy is the first to obtain approval for use in older adults in the country.
The latest development was based on the findings from a Phase III clinical trial programme in which Arexvy demonstrated 82.6% efficacy in preventing LRTD in older subjects.
The vaccine also demonstrated an efficacy of 94.6% in individuals with underlying medical issues.
Arexvy was found to be well-tolerated with a suitable safety profile.
Pain at the injection site, fatigue, myalgia and headache were among the most reported adverse events in the trials.
The company anticipates making Arexvy available in the country before the 2023-2024 peak RSV season.
The vaccine has already received approval for use in older adults in the US, Europe and the UK, with regulatory reviews underway in Japan.
GSK country medical director Marni Freeman stated: “With the approval of Arexvy, we are excited to be able to offer an option to help protect the nearly ten million Canadians aged 60 and older who are at risk of RSV disease.
“We’re hopeful that with a vaccine now available for older Canadians, the virus’s burden on our healthcare system will also be dramatically improved.”
Earlier this month, the US Food and Drug Administration granted approval for GSK’s Jemperli (dostarlimab), along with carboplatin and paclitaxel, as a monotherapy to treat primary advanced or recurrent endometrial cancer in adults.