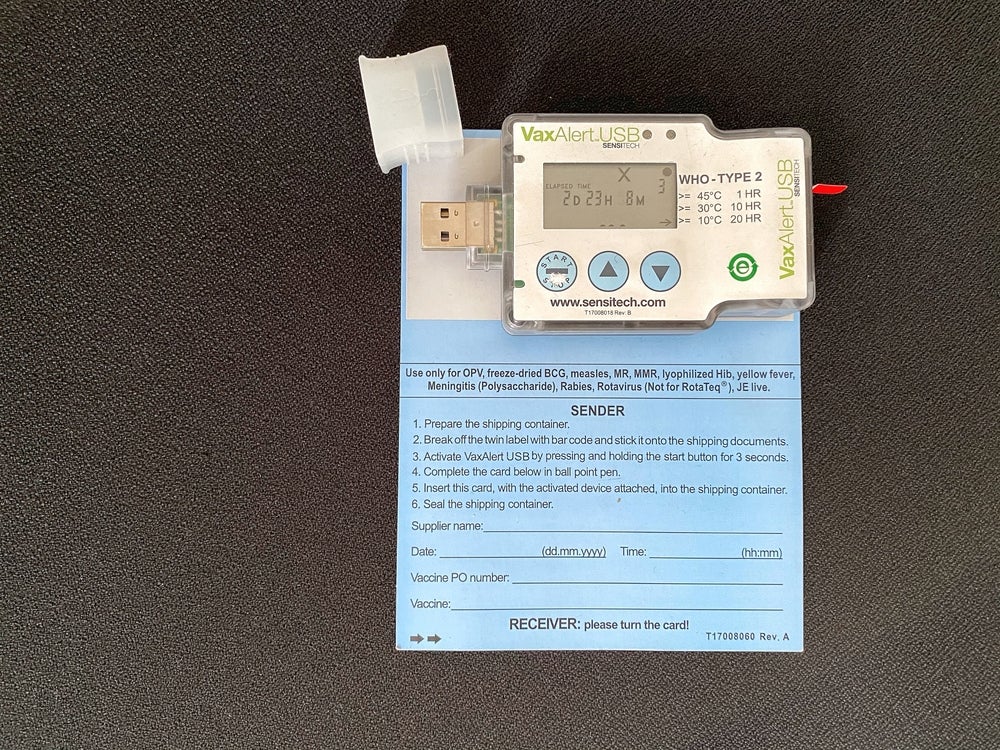
Cold chain logistics have improved after the approval of new cell therapies and a significant learning curve around transporting Covid-19 vaccines. The pandemic changed existing frameworks, introduced new players with varying expertise levels, and moved towards an efficient cold chain supply system for cell and gene therapies with an aim to boost performance and sustainability.
“Over the last decade, the cell and gene therapy sector has witnessed a huge increase in research, funding, and manufacturing, which together have led to enormous progress for this revolutionary branch of medicine,” Aloysius Chong, project director of biopharmaceutical networking group, IMAPAC, told Pharmaceutical Technology.
In July 2023, 23 new CELL AND GENE THERAPYs are in line for approval across the European Union (EU) and the United States (US), while the industry is starting to hear more regular accounts of the real-world impact of cell and gene therapies on patients, Chong says. In line with this regulatory development is a growing demand for adequate storage and transportation systems to ensure the viability and accuracy of these treatments.
“Certainly, this demand is being answered – the past decade has brought an ongoing rise in the volume of investment pumped into cold chain logistics,” says Chong. In 2020, temperature-controlled logistics accounted for nearly 18% of biopharma logistics spending, according to the Biopharma Cold Chain Sourcebook.
With the cell and gene therapy market is set to surpass $81 billion by 2029, as per GlobalData’s consensus sales and forecasts, the industry is looking at the continued advancement of cold chain logistics. Across Europe and the UK, the impact of the increasing need for cell and gene therapies has indicated how collaborative cold chain networks and partnerships can help support better access and delivery of therapies across the region.
“At the moment, the cell and gene therapy sector remains largely in its developmental stage,” adds Chong. The industry annually sees new and innovative treatments being released for patient use, yet many therapies have yet to be on any significant scale.
“All of this is changing, however, partly as a result of greater partnerships and collaboration and partly because we’re seeing years of research come to fruition,” Chong continues. “The outlook is for more treatments to be approved – meaning that continued development of cold-chain systems – in proportion with these advancements in cell and gene therapy – will be vital in the coming years,” Chong says.
Lessons from Covid-19
The pharmaceutical industry learnt from transporting Covid-19 vaccines, which changed the cold chain logistics field. Vaccine production was restricted to a limited number of manufacturing centres so effective transportation was vital to the universal fight against the outbreak, Chong says. Additionally, vaccinations needed to be administered quickly and on a never-before-seen scale.
“The challenge was unprecedented: and since vaccines contain biological materials, the precious supplies being produced required strict cold-chain transportation to ensure their viability,” Chong continues. This occurred at a time when the industry couldn’t afford to lose medicine to temperature-related decline. “All of this meant a sudden, urgent demand for cold chain technology,” Chong adds.
Cold chain manufacturers were required to quickly address several challenges. Firstly, the difficulty to maintain cold chain systems in areas with limited infrastructure. Then the hurdle of balancing the environmental impact of cold chain logistics with their importance for global healthcare. Next, the industry needed to consider the massive challenge of retaining accurate information on medical products as cold chain manufacturers scale up rapidly.
Since 2020, the industry has made practical solutions to address these difficulties through artificial intelligence (AI) analytics, streamlined supply systems, and sustainability-focused technology.
“What we have learnt most is the scope of what we can achieve: how cold chain systems can and must be part of a huge leap forward in medical history,” says Chong. The capacity to transport biological material further supports the more significant employment of decentralised clinical trials, allowing for broader geographical and economic participation.
Improvements to cold chain technology also reduce the need for the “just in time” approach to cell and gene therapy, where treatments must be manufactured on an exact timeline with their administration. The development is expected to have a positive impact on cell and gene therapy’s scalability.
Creating an efficient system
Rising demand for cold chain technology has encouraged a swathe of new manufacturers.
There are calls for increased regulation among manufacturers. “Without clear and concise guidance and stringent checks, the industry risks inviting sub-standard products that could jeopardise the viability of cell and gene materials,” Chong highlights. Ongoing dialogue between manufacturers, researchers, and healthcare professionals ensures standards remain high and cold chain logistics can reach its full potential.
While cold chain logistics can potentially open cell and gene therapies to the broader world, only some regions have the resources and infrastructure to support these systems. For example, in 2021, it was reported that only 14% of promised Covid-19 vaccines reached poorer nations globally due to many patenting, production, and supply chain issues.
“Cold chain providers must guarantee sufficient infrastructure throughout the delivery process to ensure that the system can be employed effectively,” Chong relays. Administration is another major factor paramount to an efficient cold chain supply system. Accurate inventory management, staff training, and timeline optimisation are vital in ensuring products reach patients when needed and in a viable medical state, Chong says.
“In the years to come, chain providers will have to maintain existing standards and enhance these administrative processes to match the demand for the scalability of cold chain technologies,” Chong highlights.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content