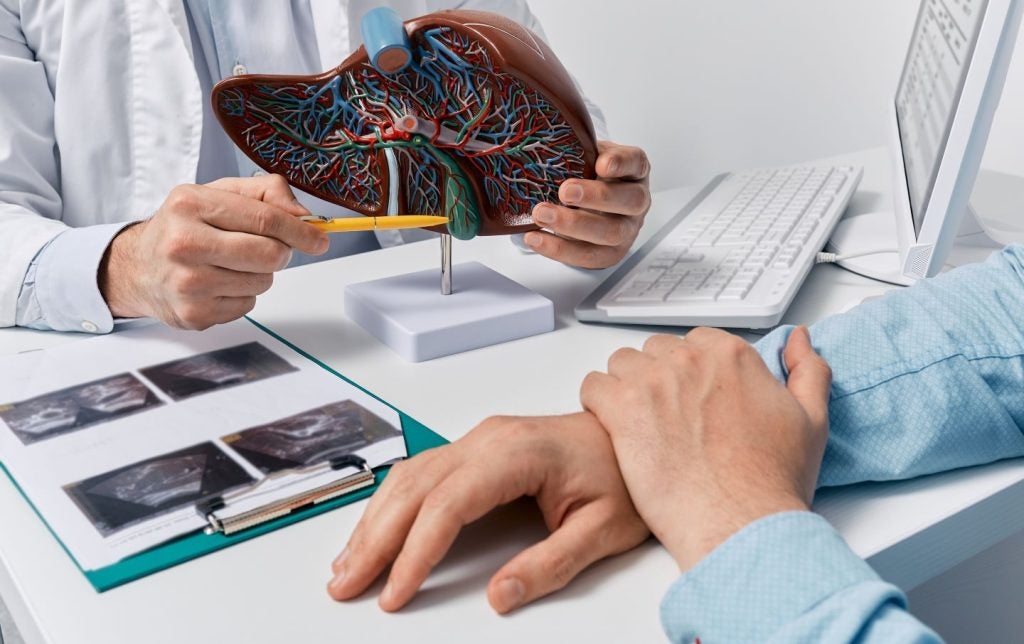
The US Food and Drug Administration (FDA) has granted fast track designation (FTD) to Biosyngen's T cell therapy BST02 for treating liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.
The designation will accelerate the clinical trial process and marketing registration for BST02.
BST02 utilises adoptive immune cell therapy technology, expanding the patient's own tumour-infiltrating lymphocytes. This approach offers potential benefits over standard tumour-infiltrating lymphocyte (TIL) therapies, including the ability to be cryopreserved and transported and a reduced requirement for high doses of interleukin-2.
In October 2023, the company received FDA approval to commence the Phase I/II clinical trials of the cell therapy.
The China National Drug Administration's Center for Drug Evaluation granted approval for the trial in December.
This marks a significant milestone as BST02 becomes the first TIL cell therapy drug for liver cancer to enter clinical stages globally.
The FTD for BST02 follows a similar designation for another Biosyngen product, BRG01, in July 2023.
Biosyngen aims to continue its research efforts to improve patient outcomes worldwide.
Biosyngen co-founder and CEO Michelle Chen expressed her gratitude to the FDA for recognising the company's fourth product.
She highlighted the company’s commitment to advancing cancer cell therapy across the globe and offering new treatments for a diverse patient population.
Chen reaffirmed the company's dedication to using its advanced technology platforms to deliver accessible therapies for patients around the world.
In September 2023, the company received FDA IND clearance for a Phase I/II trial of its therapy, BRL03, to treat lung cancer, gastric cancer and other advanced solid tumours.
BRL03 is the first T cell receptor product developed by the company to enter the clinics.