The BIOSECURE Act could impact more than 120 US biopharmaceutical drugs in development by companies partnered with Chinese contract development and manufacturing organisations (CDMOs) and biotechnology companies.
Of these, approximately half are in clinical-stage development (Phases I to III) and a third are in early-stage preclinical trials and discovery, according to leading data and analytics company GlobalData’s Pharma Intelligence Center Deals Database.
Introduced in January 2024, the BIOSECURE Act is US federal legislation that aims to prevent 'foreign adversary biotech companies' from receiving US federal funding.
The act mentions major Chinese companies WuXi AppTec, BGI, MGI, and Complete Genomics along with their subsidiaries, parents, affiliates, and successors, citing them as risks to US national security.
In addition, the bill aims to prevent US biopharmaceutical companies that collaborate with these Chinese companies 'of concern' from accessing US federal funding mechanisms such as contracts, loans, and grants.
Last month, US lawmakers revised the BIOSECURE Act, setting a deadline of 1 January 2032 for US biopharmaceutical companies to end their contracts with these Chinese companies.
According to GlobalData’s Pharma Intelligence Center Deals Database, more than 45 companies headquartered in the US have entered into contract service agreements (CSAs), licensing agreements, or partnership deals with Complete Genomics, WuXi Biologics Cayman, WuXi AppTec, or BGI Genomics, and their subsidiaries.
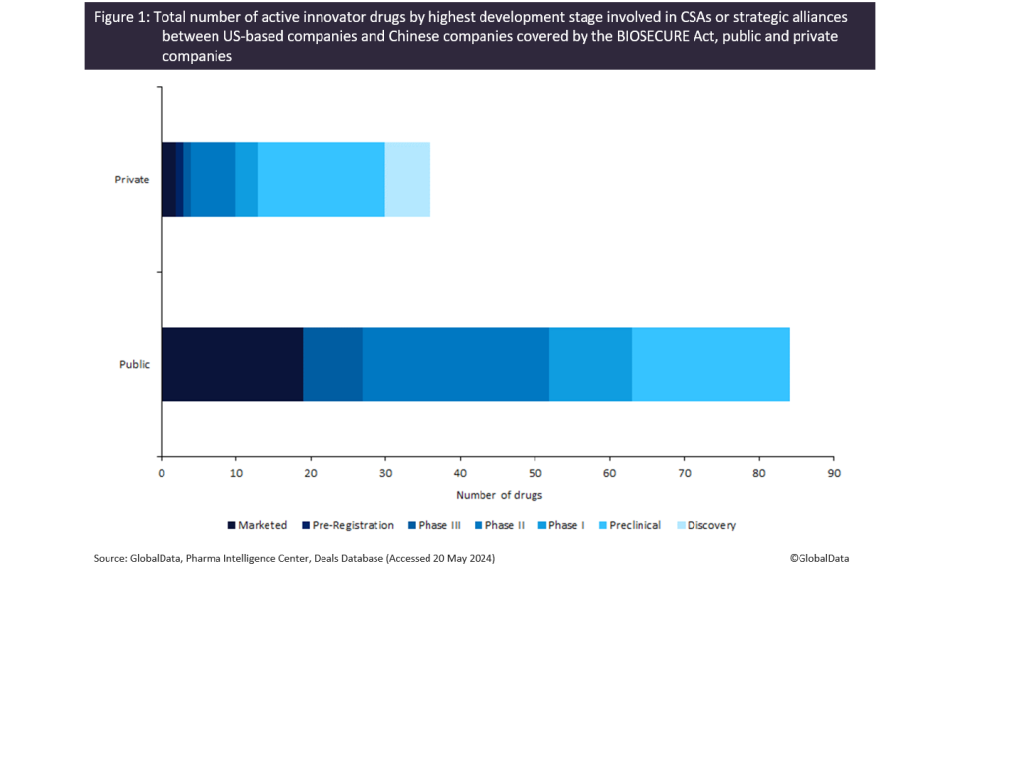
More than two-thirds of drugs that could be at stake due to the BIOSECURE Act were developed by public companies in the US, of which approximately 60% are marketed or in late clinical stage (Phase II-III) trials, as shown in Figure 1.
This demonstrates longstanding relationships between significant players in the biopharmaceutical industry and those major Chinese companies, given that it typically takes more than a decade to bring a drug to market.
Several biopharmaceutical companies, including Merck, Gilead Sciences, and Vertex Pharmaceuticals, have cited increased costs, delays in clinical trials, US Food and Drug Administration (FDA) regulatory submissions, and launch of drugs as potential impacts of the BIOSECURE Act in their company filings.
In addition, it remains to be seen whether biopharmaceutical companies with drugs in-licensed from Chinese companies 'of concern' will grapple with increased regulatory stringency from the FDA, and whether their ability to secure Medicare and Medicaid reimbursement of these drugs will be impacted.
As biopharmaceutical companies look to diversify suppliers before the 2032 deadline set by the BIOSECURE Act, CDMOs headquartered outside of China may experience increased growth over the next decade.
In particular, Indian CDMOs such as Cipla, Syngene, and Aurobindo may be well-positioned for increased growth due to their cost-effectiveness and highly skilled workforce.
However, growing concerns of US dependency on offshore suppliers may also result in a shift towards US companies selecting domestic CDMOs.