The Asia Pacific (APAC) region is leading the charge for adoptive cell therapy studies, says Scotty Chung-Siu, a managing analyst at GlobalData.
At the Annual Outsourcing in Clinical Trials conference, held from 11-12 June, Chung-Siu led a comprehensive session on adoptive cell therapies, giving an overview of the space to the audience of clinical trial professionals.
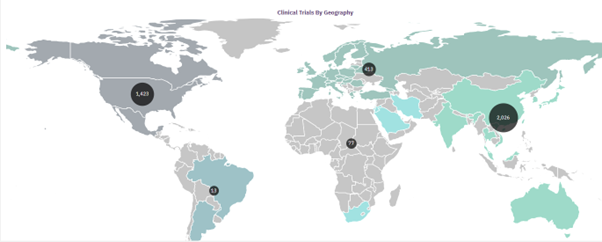
Chung Siu cited a GlobalData report that demonstrated that the number of ongoing single-country adoptive cell therapy studies in the APAC region has reached approximately twelve times the number of trials in Europe. He also highlighted that overall, there are 1,023 single country adoptive cell therapy clinical studies being run in the APAC region compared to 476 in North America.
GlobalData is the parent company of Pharmaceutical Technology.
Adoptive cell therapies are immunotherapies that use engineered cells to trigger a patient’s immune system to treat a condition. The three main types of adoptive cell therapies are tumour-infiltrating lymphocyte (TIL) immunotherapy, natural killer (NK) cell immunotherapy, and T cell immunotherapies. The last category includes several modalities such as chimeric antigen T (CAR-T) cell therapies, natural killer T cells, regulatory T cells, and T cell receptor (TCR) therapies.
Blockbuster adoptive cell therapies such as Novartis’ CAR-T therapy Kymriah (tisagenlecleucel), and Gilead Sciences’ and Daiichi Sankyo’s Yescarta (axicabtagene ciloleucel) are commercially available in some Asian countries, with the former company placing cell therapy manufacturing sites in Japan in 2020.
A February 2024 paper published in the Blood Cell Therapy journal, reported that Asia has driven 70% of global economic growth in the last two years, and estimated a compound annual growth rate for CAR-T adoption in Asia of 40%.
Chung Siu also pointed to the Bongguan, Hebei, China-headquartered Hebei Senlang Biotechnology and the Chongqing, China-based Chongqin Precision Biotech as some of the top industry sponsors, leading with 34 and 32 adoptive cell therapy trials, respectively.
The London conference held sessions discussing a wide range of topics in the field of clinical research, ranging from the UK clinical trial landscape to the use of AI in clinical trials.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.