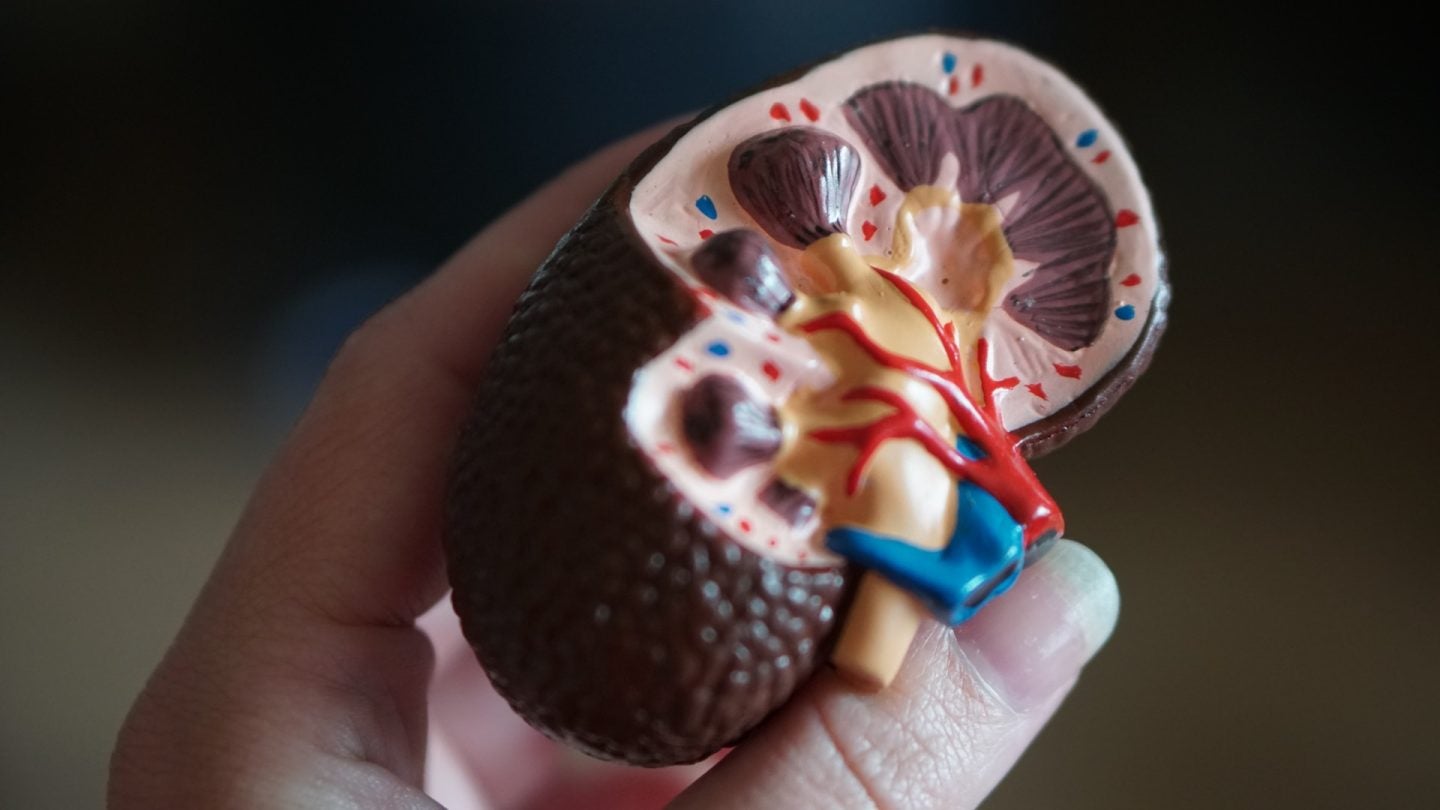
ZyVersa Therapeutics has received a patent from the European Patent Office for its VAR 200 (2-hydroxypropyl-beta-cyclodextrin) to treat patients with diabetic nephropathy/diabetic kidney disease.
Developed in the laboratories of the University of Miami in the US, VAR 200 is a cholesterol efflux mediator that is claimed to eliminate accumulated lipids from the kidney.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is currently in its Phase IIa development stage.
This asset is being evaluated to cut down the build-up of renal cholesterol and lipid that harms the filtration system of kidneys in individuals with glomerular diseases such as focal segmental glomerulosclerosis, diabetic kidney disease and Alport syndrome.
ZyVersa received a global licence to develop and market VAR 200 from L&F Research.
In June 2023, the European Patent Office issued a notice of intention to grant a patent for VAR 200.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataZyVersa Therapeutics co-founder, chairman, CEO and president Stephen Glover stated: “Approval of this patent claiming our cholesterol efflux mediator VAR 200 for use in treating diabetic kidney disease speaks to the innovative and important research conducted by Dr Fornoni and her team involving removal of excess cholesterol and lipids that damage the kidneys’ filtration system.
“Strengthening our intellectual property portfolio for VAR 200 and expanding our patent protection and exclusive rights into additional geographic regions will further enable ZyVersa to increase shareholder value as VAR 200 advances into clinical trials, which are planned for initiation in the fourth quarter of this year.”




