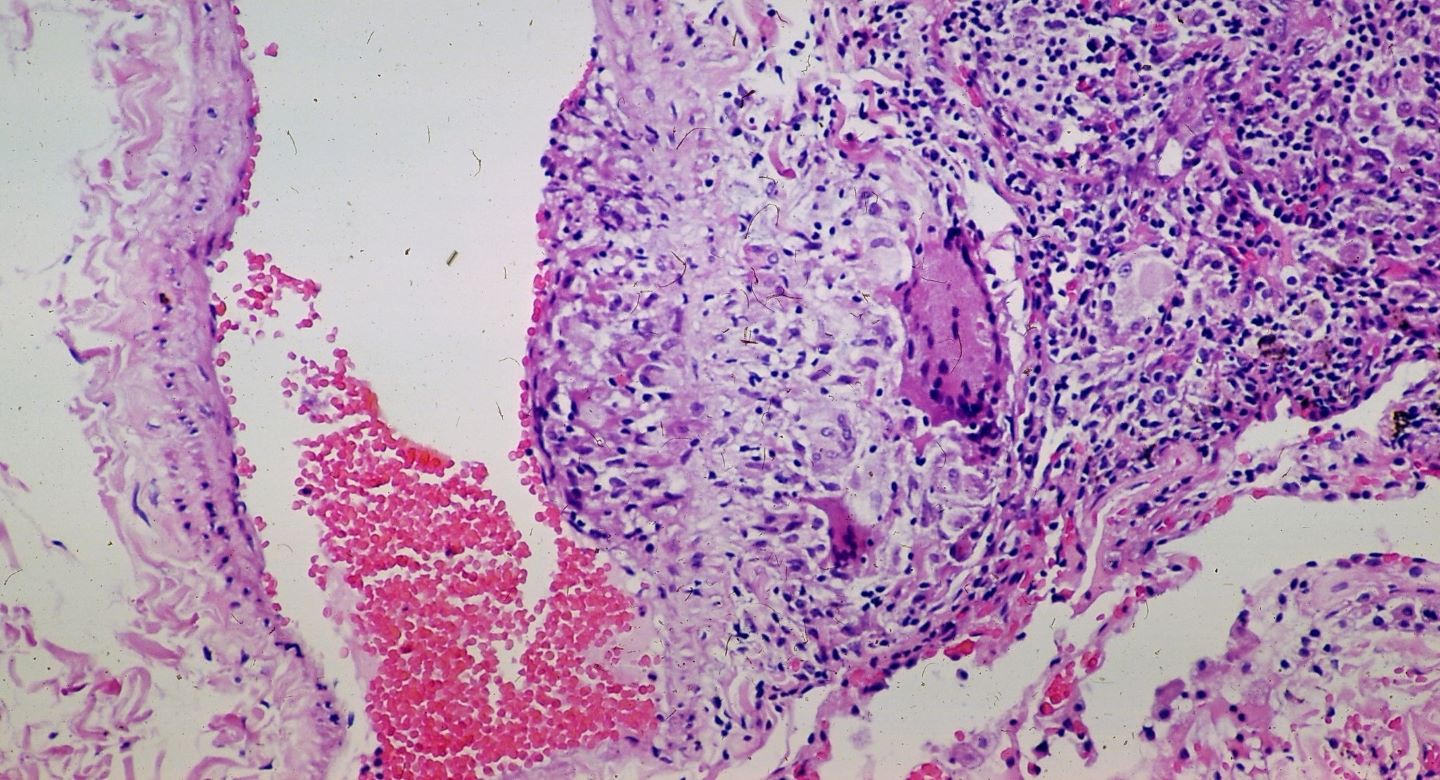
Xentria has entered an exclusive multi-year licensing agreement with Meitheal Pharmaceuticals for the marketing of Xentria’s new biologic, XTMAB-16, to treat pulmonary sarcoidosis in North America.
XTMAB-16 is a lead candidate of Xentria and an anti-TNFα monoclonal antibody.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Meitheal will obtain sole rights to market the biologic in North America while Xentria will oversee its clinical development.
Xentria will hold the rights to market the product in all other regions, and will be entitled to $45m in upfront payments and up to $35m on regulatory submission and approval.
The company is also eligible to receive milestone-based royalty payments exceeding $600m for licensing.
The antibody will be tested in Phase II clinical trials in 2023 to analyse its potential to reduce the inflammatory response associated with pulmonary sarcoidosis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn 2022, Xentria concluded a Phase I trial of the biologic in healthy subjects.
Xentria COO Kirsten Anderson stated: “The collaboration will leverage the strengths of both companies to accelerate the development of XTMAB-16, which could emerge as one of a few therapeutics in development specifically for treating pulmonary sarcoidosis, in contrast to symptomatic steroid treatment as the current standard of care.
“The partnership also validates compelling pre-clinical data and the potential of XTMAB-16 to fill the existing treatment gap in pulmonary sarcoidosis as we enter the first trial in patients living with the condition.”


