Vidac Pharma has secured €600,000 ($645,651) in additional funding to support the clinical development of its two lead cancer candidates.
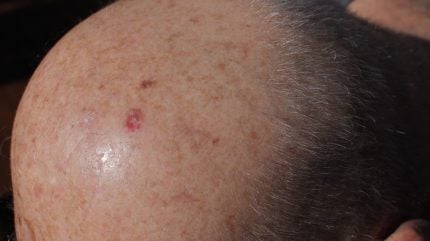
The funding from existing investors, announced at the company’s recent shareholder meeting, will enable Vidac to initiate a new Phase IIb trial for its lead candidate VDA-1102 (tuvatexib) ointment. The hexokinase 2 inhibitor is being investigated in advanced cases of actinic keratosis, a skin condition that can progress to cutaneous squamous cell carcinoma (SCC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Alongside VDA-1102, the UK-based biotech continues preclinical studies of VDA-1275. Both candidates target the metabolic pathways of cancer cells by disrupting hexokinase 2 interactions with mitochondrial channels, aiming to reverse the high-energy demands of tumours and promote cancer cell death. VDA-1275 is being developed as a systemic drug for the treatment of solid tumours.
Interim data from a Phase IIa study (NCT02844777) released in January 2023 showed a 56% objective response rate (ORR), with 22% complete responses (CR), observed between eight and 12 weeks. This compares to the standard-of-care treatment mechlorethamine, which has a 13% CR and a longer median response time of 26 weeks. Final results are expected in Q4 2024.
Vidac has also completed a Phase IIb clinical trial (NCT03538951) for VDA-1102 in actinic keratosis. In a post-hoc analysis, the therapy demonstrated a 40% complete clearance of lesions and an 80% overall reduction in lesion count. The study also indicated that patients with advanced actinic keratosis responded more favourably to the treatment than those with less severe forms. Based on these findings, the upcoming Phase IIb trial will focus specifically on patients with advanced actinic keratosis.
Unlike conventional actinic keratosis treatments that directly destroy abnormal cells, through topical therapies, cryotherapy, or photodynamic therapy, VDA-1102 works by reprogramming cancer cell metabolism. This selective approach aims to spare healthy cells, and in the Phase IIa trial, resulted mostly in mild, localised side effects, with one moderate case. Standard actinic keratosis treatments, by contrast, can cause skin inflammation, scaling, or burning, and are often invasive.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the announcement accompanying the funding, Vidac’s CEO Max Herzberg said: “I want to thank our board, advisers, team and shareholders for their support for our entirely new approach in oncology, with drugs that reverse the supercharged metabolism of cancer cells.”
In June 2024, Almirall’s Klisyri (tirbanibulin) secured FDA-expanded approval to treat larger actinic keratosis areas to up to 100cm² from the previous maximum of 25cm². Klisyri works by blocking cell division and killing abnormal cells. The most common side effects are local skin reactions, including erythema (reddening of the skin), flaking/scaling, crusting, swelling and the formation of sores and ulcers.
Innovations in the pharma and medical device landscape are looking to expedite the diagnosis of skin cancers. In July 2023, the National Cancer Institute (NCI), part of the US National Institutes of Health (NIH), awarded $2m in funding to Enspectra Health through the Small Business Innovation Research (SBIR) programme. The grant will fund the development of deep learning algorithms that can predict which actinic keratosis skin lesions are likely to turn into SCC.




