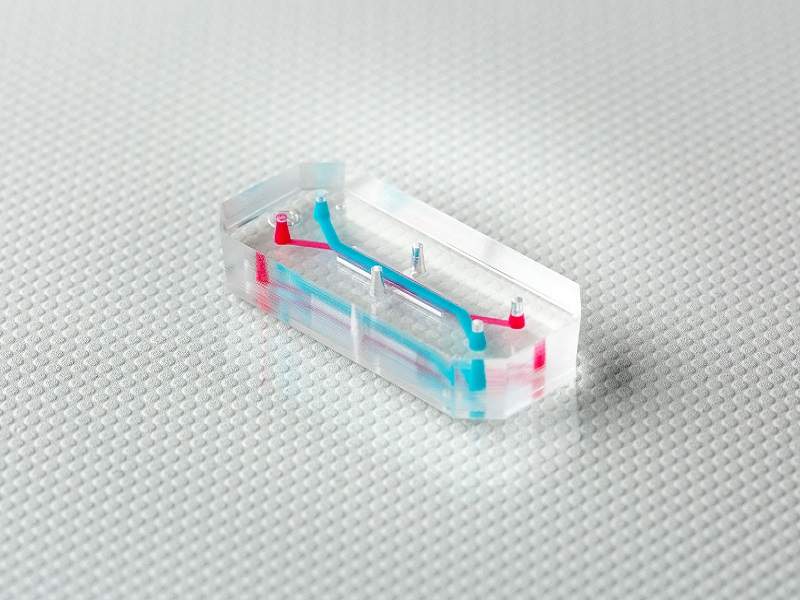
Roche and Takeda have announced that they will be using organ-chip technology in their R&D programmes through partnerships with Emulate, the biotech startup behind organs-on-a-chip.
Emulate and Roche have formed a three-year partnership to use organ-chips for testing the efficacy and safety of new antibody therapeutics and combination therapies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Working in a jointly-run lab, researchers hope to use the chips to gain further insight into disease mechanisms and to increase predictability and early detection of biomarkers. The collaboration will initially use lung-chips and brain-chips with the possibility of using other organ-chips.
The partnership with Takeda will use Emulate’s intestine-chip in the R&D of drugs to treat gastrointestinal (GI) diseases. The intestine-chip can be used to recreate the mechanisms of the intestinal lining, which is understood to be associated with many GI diseases.
Financial details of the partnerships were not disclosed.
An organ-on-a-chip is a microfluidic cell culture chip that simulates the activities, mechanics and physiological response of entire organs and organ systems. Animal testing often fails to predict how drugs will perform in patients, and so the new technology has the potential to reduce animal testing and implement more personalised testing of new drug candidates. They may eventually abolish the need for animals in drug development and toxin testing.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAlthough the technology is relatively new, it is poised to move into mainstream drug development. Currently, 30% of drugs do not make it past trials because they are too risky to test on humans. By accurately replicating the specific effects of a drug on an organ or organ system, results can be observed more quickly and at a much lower cost than through animal testing, and without the safety concerns that often prevent drugs from progressing to human trials.
The technology also offers a solution to the ever-present ethical dilemmas surrounding the use of animals in drug testing.
Organ-chips have received a high level of interest from the pharmaceutical industry, with Emulate already in partnership with Johnson & Johnson and Merck. The organs-on-chips market is expected to be worth around $1 billion in the next decade.
In April 2017, the US Food and Drugs Administration announced a multi-year research and development agreement with Emulate to evaluate the company’s organ-chip technology.
President and chief scientific officer of Emulate Geraldine Hamilton said: “Our approach is to start with the patient and end with the patient by using patient-derived cells with organ-chips. As we produce more human-relevant data with our organ-chips, we look forward to making a positive impact on informing R&D decisions, reducing drug candidate attrition in human clinical trials, and helping to deliver better and safer medicines to patients.”




