
Swiss clinical-stage pharmaceutical company PIQUR Therapeutics has received orphan drug designation from the European Medicines Agency (EMA) for its lead compound, PQR309, indicated to be used on patients with diffuse large B-cell lymphoma (DLBCL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PQR309 is an oral, brain-penetrant, dual inhibitor of the PI3K/mTOR pathway, which is reported to become activated in 60%-80% of human cancers.
DLBCL is an aggressive form of lymphoma and also forms one of the most common types of non-Hodgkin lymphoma (NHL).
The disease primarily affects older people but can also occur in children and young adults in rare cases.
About 10%-15% of DLBCL patients exhibit refractory disease and an additional 20%-25% report relapse of the disease after initial response to therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPIQUR senior regulatory affairs manager Claudia Pluess said: “The EMA orphan drug designation for PQR309 in DLBCL is another important regulatory milestone, validating the potential therapeutic use of PQR309 in DLBCL.
PQR309 has exhibited both preclinical activities in various tumour models and clinical activity in Phase 1 and 2 studies.
PIQUR CEO Dr Vladimir Cmiljanovic added: “PIQUR will continue to work with physicians and regulatory agencies to further define the clinical development strategy to bring a potential new treatment option to patients suffering from this disease.”
PIQUR has recently also received orphan drug designation from the FDA for PQR309 for the treatment of primary CNS lymphoma (PCNSL).
The EMA orphan drug designation is a status awarded to a medicine indicated to be used on a rare condition.
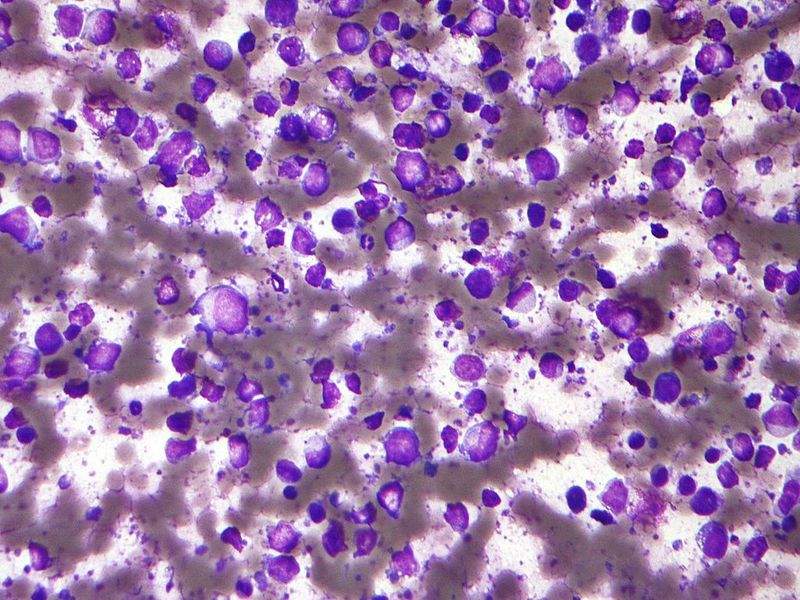
Image: Micrograph of a diffuse large B-cell lymphoma. Photo: courtesy of Nephron.




