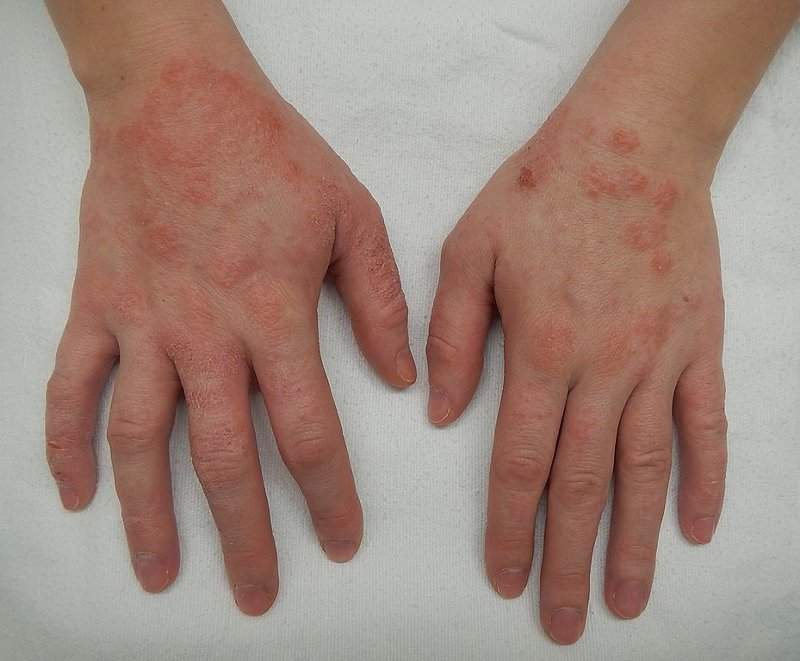

The US Food and Drug Administration (FDA) has approved Anacor Pharmaceuticals’ Eucrisa (crisaborole) ointment for the treatment of mild-to-moderate eczema (atopic dermatitis) in patients aged two and older.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Chronic inflammatory skin disease Atopic dermatitis is often referred to as ‘eczema' and is the most common of the many types, with onset typically occurring in childhood and possibly continuing through to adulthood.
FDA Center for Drug Evaluation and Research (CDER) Office of Drug Evaluation III deputy director Amy Egan said: "Today’s approval provides another treatment option for patients dealing with mild-to-moderate atopic dermatitis."
The disease is caused by a combination of genetic, immune and environmental factors. The skin develops red, scaly and crusted itchy bumps.
Scratching will lead to swelling, cracking, ‘weeping' clear fluid, and the skin will also become rough and thick.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe safety and efficacy of the phosphodiesterase 4 (PDE-4) inhibitor Eucrisa ointment were established in two placebo-controlled trials.
A total of 1,522 participants aged two to 79 years with atopic dermatitis participated in the trials.
Serious side effects of Eucrisa include hypersensitivity reactions and most the common side effect is application site pain, including burning or stinging.
The ointment is not recommended for patients who have had a hypersensitivity reaction to its active ingredient, crisaborole.
Image: Dermatitis or eczema of the hands. Photo: courtesy of James Heilman, MD.


