
EUSA Pharma has secured European Commission (EC) approval for the antibody ch14.18/CHO, (dinutuximab beta) to be used in treating high-risk neuroblastoma in patients older than a year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The approval makes dinutixumab beta the only permitted immunotherapy in Europe for high-risk neuroblastoma treatment.
Dinutuximab beta is a monoclonal chimeric antibody that targets a specific antigen GD2 on neuroblastoma cells.
Neuroblastoma is one of the most common forms of solid tumours and primarily affects children under five years of age.
Every year, around 1,200 children are diagnosed with neuroblastoma in Europe.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is a rare form of cancer that arises from neural crest cells, which are responsible for the foetal development of the nervous system.
The disease spreads fast and most of the cases are diagnosed in the advanced stages, which are recognised as ‘high-risk’.
University of Southampton associate professor and paediatric oncology consultant Dr Juliet Gray said: “As a clinician working in a highly specialised disease area with limited treatment options, I greatly welcome the availability of this targeted immunotherapy treatment that offers improved results for high-risk neuroblastoma patients used alone or in combination with existing therapies.”
Dinutuximab beta has been investigated in more than 1,000 patients during clinical trials.
The approval is expected to provide hope to numerous children affected by neuroblastoma.
EUSA Pharma CEO Lee Morley said: “We are delighted that the EC has recognised the urgent need to accelerate approval of dinutuximab beta in order to provide an effective and targeted treatment for this debilitating disease.
“EUSA Pharma in partnership with Apeiron and SIOPEN has believed strongly in the potential of this treatment throughout the clinical trial process and today’s announcement is the final acknowledgement of its value to address the serious unmet need of children and their families affected by high-risk neuroblastoma.”
EUSA is also planning to file for dinutuximab beta approval in the US later this year.
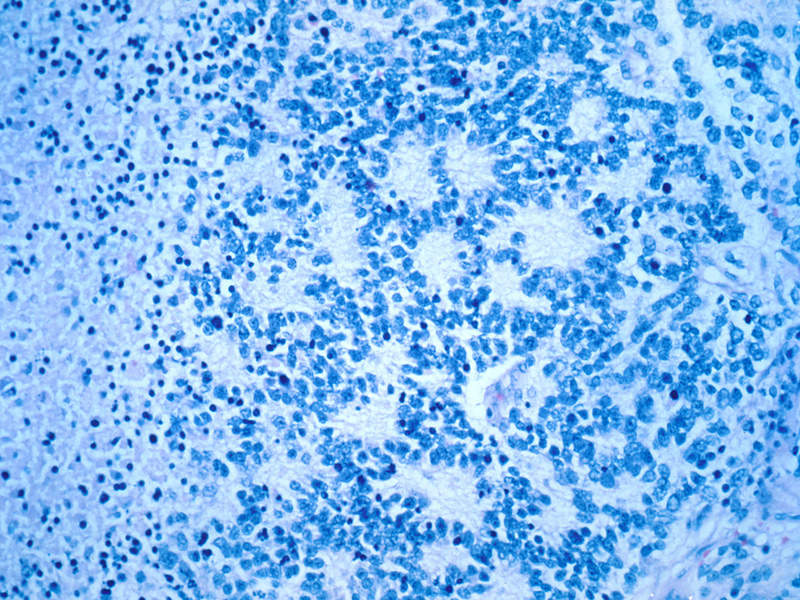
Image: Microscopic view of a typical neuroblastoma with rosette formation. Photo: courtesy of Dr Maria Tsokos, National Cancer Institute.




