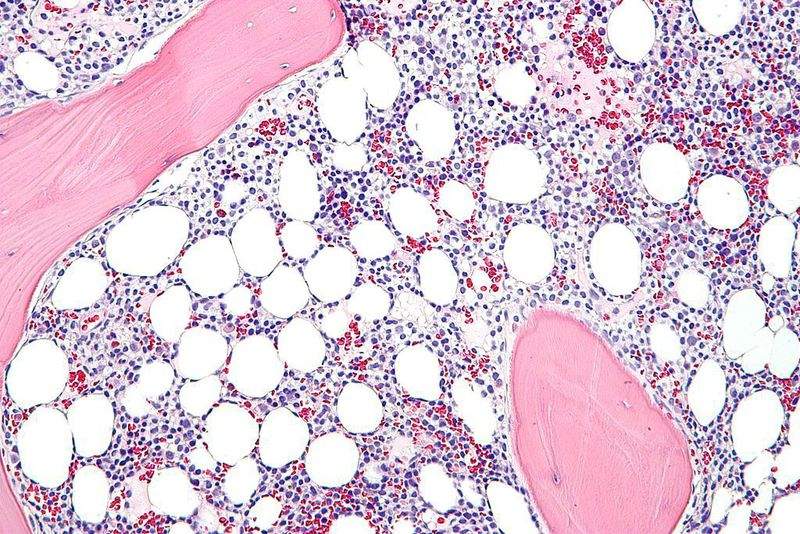
AstraZeneca and its research and development arm MedImmune have obtained approval from the US Food and Drug Administration (FDA) for the use of Lumoxiti to treat adults with relapsed or refractory hairy cell leukaemia.
The approval covers treatment of patients who had at least two previous systemic therapies, including treatment with a purine nucleoside analogue. Patients with severe renal impairment are warned against using the newly approved drug.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Lumoxiti (moxetumomab pasudotox) is a CD22-directed cytotoxin. It consists of the CD22 binding portion of an antibody connected to a truncated bacterial toxin, which inhibits protein synthesis and induces apoptotic cell death.
AstraZeneca Oncology business unit global head Dave Fredrickson said: “Today’s FDA approval of Lumoxiti represents a significant milestone for people living with hairy cell leukaemia, a rare blood cancer that can result in serious and life-threatening conditions.
“For patients, this approval provides the first FDA-approved medicine for this condition in more than 20 years.”
The FDA review comes from the single-arm, open-label Phase III 1053 clinical trial that evaluated Lumoxiti monotherapy in 80 subjects. Primary endpoint of the trial was durable complete response.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataResults showed that 30% of trial participants administered with the drug had a durable complete response and 75% of subjects experienced an overall response.
Patients administered with Lumoxiti had capillary leak syndrome (CLS) and haemolytic uraemic syndrome (HUS), including life-threatening cases of the conditions.
The most common Grade 3 or 4 adverse reactions observed in the trial were HUS, hypertension and febrile neutropenia. HUS was found to be responsible for discontinuation in 5% of cases.
The drug was approved following a priority review process.




