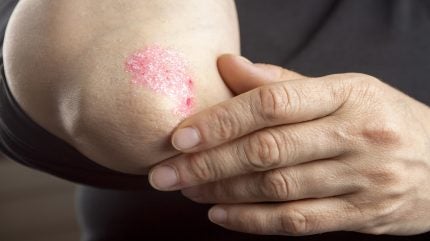
LEO Pharma has submitted a New Drug Application (NDA) to China’s National Medical Products Administration for Enstilar, targeting the treatment of adult patients with plaque psoriasis.
Enstilar is an aerosol spray foam combining calcipotriol and betamethasone, designed to improve upon the existing Daivobet ointment, which is currently a standard treatment in China.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The submission follows a series of milestones for LEO Pharma in the EU and the US, with China potentially becoming the largest market for Enstilar.
The NDA is supported by a Phase III trial involving 604 adult Chinese subjects with stable plaque psoriasis.
This trial showed Enstilar to be superior to Daivobet ointment, achieving both primary and secondary endpoints.
The Center for Drug Evaluation (CDE) in China will now validate and fully evaluate the NDA. The regulatory review process is anticipated to be completed in the first half (H1) of 2026.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLEO Pharma China general manager Byron Yin said: “LEO Pharma strives to enhance patient care and elevate quality of life for those living with complex skin conditions like plaque psoriasis.
“Today’s submission is a crucial step towards offering a much-needed new treatment option in our region, in addition to the existing portfolio offering from LEO Pharma China.”
Enstilar is already indicated in the EU for up to four weeks of treatment for psoriasis vulgaris in adults, with suitable candidates eligible for long-term maintenance.
Recently, the European Commission authorised Leo Pharma’s Anzupgo cream for treating adults with moderate to severe chronic hand eczema.




