The US Food and Drug Administration (FDA) has accepted for review the resubmitted supplemental Biologics License Application (sBLA) for Sanofi and Regeneron’s Dupixent (dupilumab) in chronic spontaneous urticaria (CSU).
The application seeks to expand Dupixent’s use to treat adults and adolescents (aged 12 and older) with CSU inadequately controlled by H1 antihistamine therapy. The FDA’s target decision date is 18 April 2025.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dupixent, a fully human monoclonal antibody, inhibits the signalling of IL-4 and IL-13 pathways and is different from other immunosuppressants used to treat this condition. The resubmission builds on data from the LIBERTY-CUPID Phase III clinical trial (NCT04180488), which included three studies (Study A, Study B, and Study C). The sBLA adds pivotal results from Study C, conducted in biologic-naive patients with uncontrolled CSU who were receiving standard-of-care antihistamines.
Study C met both its primary and key secondary endpoints, demonstrating that Dupixent significantly reduced itch and urticaria activity (hives and itch severity), aligning with earlier results from Study A. Safety data across the LIBERTY-CUPID programme were consistent with Dupixent’s established safety profile, with common adverse events including injection site reactions and Covid-19 infections.
Dupixent has already been approved for CSU in Japan and the United Arab Emirates (UAE) and is under regulatory review in the European Union. Outside of Japan and the UAE, the safety and efficacy of Dupixent in CSU remain under evaluation.
The potential new indication for CSU comes as Regeneron and Sanofi continue to expand Dupixent’s market presence. In September 2024, the FDA approved Dupixent for chronic obstructive pulmonary disease (COPD), marking its sixth indication and making it the first biologic approved for COPD. Dupixent is also approved for conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDupixent generated $11.3bn (€10.7bn) in global sales in 2023, as per Regeneron’s financials. Projections from GlobalData’s Pharma Intelligence Center suggest revenue could reach $23.6bn by 2030.
GlobalData is the parent company of Pharmaceutical Technology.
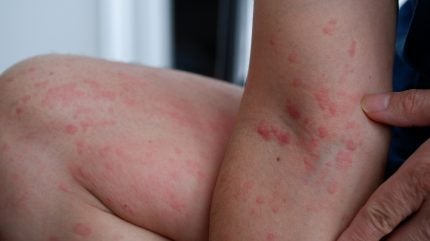
CSU is a skin condition characterised by persistent, itchy hives that last six weeks or longer. While antihistamines and anti-inflammatory treatments are used to manage symptoms, there is an unmet need for more effective options in patients with uncontrolled disease.
Meanwhile, Novartis is advancing its CSU pipeline with promising data for its investigational drug, remibrutinib. Results from the Phase III REMIX-1 (NCT05030311) and REMIX-2 data (NCT05032157) released in May 2024 showed that almost half of patients were completely free of itch and hives as assessed at week 52 using urticaria activity scores. Novartis said it plans to submit remibrutinib for regulatory approval in the second half of 2024.
The last paragraph has been updated to clarify that the drug Novartis plans to submit for regulatory approval is remibrutinib.




