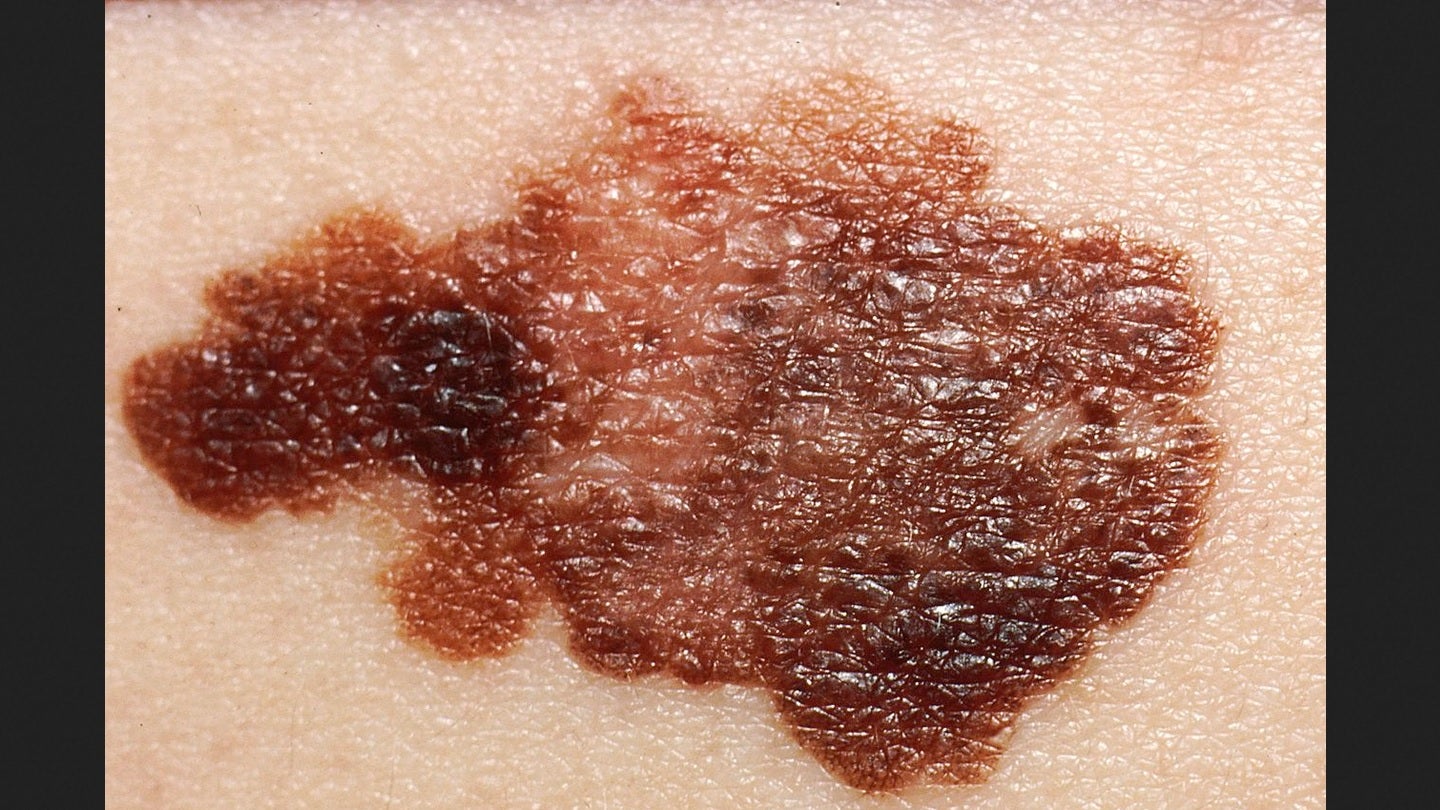
The US Food and Drug Administration (FDA) has accepted Iovance Biotherapeutics’ biologics license application (BLA) for lifileucel to treat advanced melanoma.
Lifileucel is a tumour infiltrating lymphocyte (TIL) therapy developed to treat advanced melanoma patients who advanced on or after prior anti-PD-1/L1 therapy and targeted therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been granted priority review by the FDA, with a target action date of 25 November this year, under the Prescription Drug User Fee Act (PDUFA).
No potential review concerns have been identified by the FDA following an initial evaluation.
Iovance interim president and CEO Frederick Vogt said: “The BLA acceptance is a significant milestone in our mission to deliver lifileucel as the first individualised, one-time cell therapy for a solid tumour.
“The FDA’s commitment to a six-month priority review validates the unmet need and urgency for new treatment options for patients with advanced melanoma who have progressed on or after standard of care therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We look forward to continuing our collaboration with the FDA during the BLA review cycle, while continuing to execute our pre-commercialisation activities and advancing our robust TIL pipeline.”
The company submitted the BLA in March this year. The BLA submission for lifileucel was based on positive data from the C-144-01 clinical study, which included patients with advanced melanoma.
The randomised phase III TILVANCE-301 trial in frontline advanced melanoma can support full approval, if lifileucel is granted accelerated approval.




