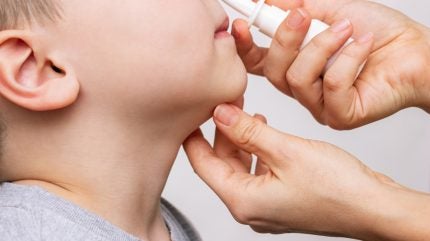
The US Food and Drug Administration (FDA) has approved ARS Pharmaceuticals’ neffy 1mg (epinephrine nasal spray) for treating type one allergic reactions including anaphylaxis in paediatric patients aged four years and above weighing between 15kg and 30kg.
Neffy’s needle-free administration allows for an instantaneous epinephrine dose through a nasal spray without the need for a nasal hold period.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The approval is supported by data from clinical trials, including pharmacodynamic and pharmacokinetic responses in both paediatric subjects and adults that were consistent with those of epinephrine injection products.
ARS Pharmaceuticals CEO, president and co-founder Richard Lowenthal stated: “Today’s FDA approval of neffy 1mg marks a major milestone towards our efforts to transform the management of severe allergic reactions.
“With nearly four out of 10 US epinephrine prescriptions written for children under the age of 18 — and nearly a third of those for children weighing 15 to 30 kilograms — we believe neffy 1mg will improve access to a needle-free option for the treatment of severe allergies and reduce hesitation in treating this vulnerable group. It will also eliminate risks like accidental needle injuries to children or caregivers.”
The spray can be utilised by paediatric patients as young as 10 by adhering to provided instructions.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEven individuals without training, such as teachers or babysitters, can administer the spray effectively.
With a shelf life of two years at room temperature, the spray can withstand temperatures up to 122°F for three months.
If the spray is inadvertently frozen, it can be thawed without affecting its reliability or quality.
The latest approval comes after the agency’s approval for neffy 2mg for children and adults weighing 30kg and above in August 2024.
In the same month, the European Commission approved EURneffy in the European Union.
The company anticipates that neffy 1mg will be available in the US by the end of May 2025.
In July 2022, ARS Pharmaceuticals signed a definitive merger agreement with Silverback Therapeutics in an all-stock transaction.




