The US Food and Drug Administration (FDA) has approved Sanofi’s Cablivi (caplacizumab-yhdp) to treat adults with acquired thrombotic thrombocytopenic purpura (aTTP), a rare blood clotting disorder.
Cablivi is a nanobody-based drug designed to target the von Willebrand factor (vWF) protein associated with blood haemostasis. It prevents the protein’s interaction with platelets to inhibit formation of blood clots.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The drug was developed by Belgian pharmaceutical firm Ablynx, which was acquired by Sanofi last year.
It is Sanofi’s first nanobody-based medicine and the first FDA-approved therapy specifically indicated to treat aTTP. The indication covers use of the drug along with plasma exchange and immunosuppressive therapy.
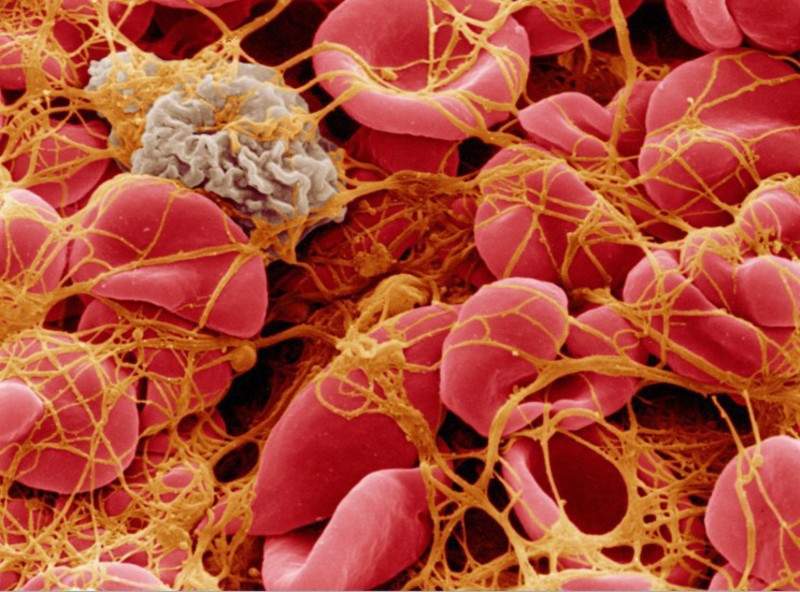
aTTP is a life-threatening, autoimmune blood disorder characterised by extensive blood clots in the small blood vessels throughout the body. The clots hinder oxygen and blood supply to the major organs, resulting in strokes, heart attacks, brain damage or death.
Commonly, patients have to undergo daily plasma exchange, which involves use of a machine that extracts blood from the body, mixes with donated plasma and returns it to the body.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe FDA noted that use of this therapy in combination with immunosuppressive drugs for days or even weeks could still lead to diseases recurrence.
FDA Oncology Center of Excellence director Richard Pazdur said: “Cablivi is the first targeted treatment that inhibits the formation of blood clots. It provides a new treatment option for patients that may reduce recurrences.”
The regulatory agency’s approval is based on data obtained during the pivotal Phase III HERCULES clinical trial conducted in 145 adults experiencing an aTTP episode.
During the trial, combination of Cablivi with plasma exchange and immunosuppressive therapy was compared to placebo, plasma exchange and immunosuppressive therapy combination.
Results showed significantly shorter time to platelet count response with the drug combination.
The drug also demonstrated decrease on a composite endpoint of aTTP-related death, disease recurrence or a major thromboembolic event.
However, Cablivi comes with a warning about the risk of severe bleeding.
The drug is set to be commercially launched in the US by the end of first quarter this year with a list price of $270,000.
In September last year, the European Commission (EC) granted approval to market the drug in the European Union (EU) for treating aTTP episodes.




