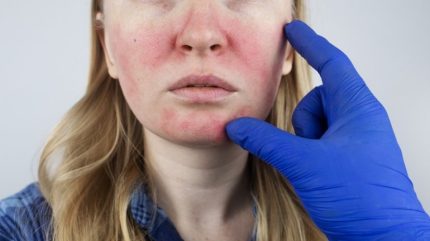
The US’ Food and Drug Administration (FDA) has approved Journey Medical’s Emrosi, the brand name for its 40mg minocycline hydrochloride extended-release capsules, to be used in the treatment of dermatological condition rosacea.
Developed in collaboration with Dr Reddy’s Laboratories, Emrosi – previously known as DFD-29 – is supported by positive data collected over two 16-week Phase III clinical trials. The results were shared in July 2023 and showed that DFD-29 successfully met all co-primary and secondary endpoints, establishing both safety and efficacy in comparison to placebo and current standard-of-care treatment Oracea, owned by Galderma Laboratories.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Journey Medical now intends “to establish Emrosi as a new standard of care in the treatment of rosacea.” This is according to CEO Claude Maraoui, who believes the drug “has the potential to become the best-in-class oral medication to treat the condition”.
Journey has reported that it is in the process of completing the manufacturing of Emrosi for the US market. It anticipates that the initial supply will be available to adult rosacea patients in the first half of 2025.
The launch will mark a change in the treatment landscape. GlobalData’s 2022 ‘Rosacea Marketed and Pipeline Drugs Assessment, Clinical Trials and Competitive Landscape‘ report stated that Galderma was “the most prominent player in the rosacea space, offering a comprehensive portfolio of solutions”. However, it also warned that with “no presence in the late-stage pipeline and with its existing marketed products widely genericised, the company may lose its position in the rosacea market”.
This balance is perhaps tipping, and Journey Medical – which GlobalData called a “less prominent player” in 2022 – is making a bid for the top spot in rosacea treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGlobalData is Pharmaceutical Technology’s parent company.
Rosacea is a chronic inflammatory skin condition, which causes reddening of the skin, lesions and ‘spider veins’, usually around the nose and cheeks. Early symptoms may come and go, but the condition usually becomes more prominent over time. According to Yale Medicine, around 5% of the global population is affected, and untreated rosacea will progress over time, eventually leading to more severe symptoms such as facial swelling, thickened skin and eye inflammation.
Rosacea treatment Emrosi will mark the latest offering in Journey Medical’s portfolio of dermatological treatments. Others include Accutane, Ximino and Amzeeq (all for varying severities of acne) and Zilxi, a topical rosacea treatment.




