The US Food and Drug Administration (FDA) has approved SpringWorks Therapeutics’ Gomekli (mirdametinib) to treat neurofibromatosis type 1 (NF1), a type of rare genetic disorder.
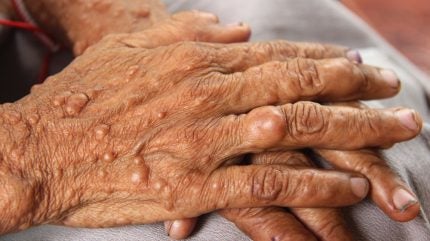
Both adult and paediatric patients with NF1 who have symptomatic plexiform neurofibromas (PN) that cannot be surgically removed will be eligible for the treatment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
NF1 is a genetic disorder that currently affects approximately 100,000 children and adults in the US. The NF1 gene codes for neurofibromin, a protein that acts as a tumour suppressor. Patients with the genetic disorder have a 30%-50% lifetime risk of developing PN, which are tumours that grow along the peripheral nerve sheath and are difficult to surgically remove. They can cause skin disfigurement, pain and functional impairment.
AstraZeneca’s Koselugo (selumetinib) became the first drug approved for NF1-PN in the US in 2020, although it is only indicated for paediatric patients aged two years and older. SpringWorks estimates that around 40,000 people in the US, both children and adults, live with NF1-PN.
Both Gomekli and Koselugo are kinase inhibitors that target mitogen-activated protein kinase kinases 1 and 2. These enzymes are responsible for cell growth and are overactive in patients with NF1.
SpringWorks’ drug demonstrated objective response rates of tumour volume reduction in 41% and 52% of adults and children respectively in the Phase 2b ReNeu trial (NCT03962543). Among those who responded to the treatment, 88% of adults and 90% of children had sustained positive effects after a year. Gomekli’s safety and tolerability profile is described as “manageable”, with the most common adverse events across adults and children being vomiting, rash, and diarrhoea.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLead trial investigator Dr Christopher Moertel said: “It was very encouraging in the ReNeu trial to see that Gomekli provided deep and durable responses, with a manageable safety profile that enabled patients to stay on therapy. This approval represents an important advance, especially for adults who previously did not have an approved treatment.”
Gomekli is available in capsules or oral suspension and Connecticut-based SpringWorks expects to distribute the treatment in the US within two weeks. The drug is also currently under regulatory review in Europe, with a decision expected later this year.
Due to Gomekli’s rare paediatric designation, SpringWorks received a priority review voucher upon approval. This voucher allows the company to fast-track a future candidate of its choice through FDA review, meaning a market placement of four months sooner than usual. SpringWorks could also sell the asset for a quicker cash injection – sales of vouchers have reached more than $100m in recent years.
The FDA approval comes at a time when German drugmaker Merck KGgA is eyeing SpringWorks as a potential acquisition. Originally reported by Reuters, Merck confirmed it is in advanced discussions with the Gomekli developer though “no legally binding agreement has been entered into”. If an agreement is reached, the takeover could happen within a few weeks. SpringWorks share price soared by 34% following the disclosure, bringing its market cap to exceed $4bn.




