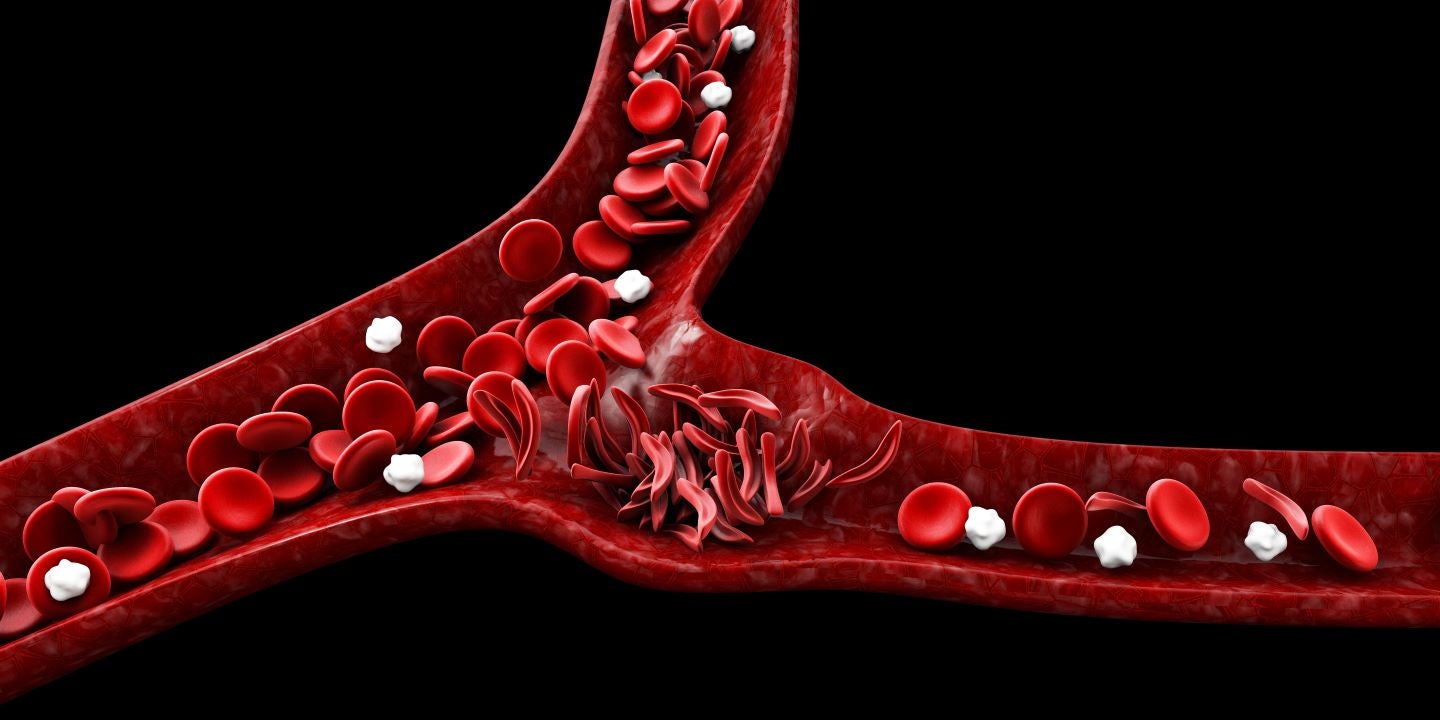
Emmaus Life Sciences has received the Oman Ministry of Health’s marketing authorisation for Endari (L-glutamine oral powder) for the treatment of sickle cell disease in patients aged five years and above.
The treatment reduces the serious complications associated with sickle cell disease in both adult and paediatric patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Almost 3,000 individuals in Oman have sickle cell disorders and approximately 6% carry the gene for the ailment.
The treatment has already received marketing approvals in Israel, Kuwait, Qatar, Bahrain, the UAE and the US.
Endari is offered on a named individual or early access basis in the Netherlands, Saudi Arabia and France.
The company plans to seek further approvals for the treatment in other regions with high sickle cell disease prevalence.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEmmaus focuses on discovering, developing and commercialising new therapies, including those intended to treat rare and orphan diseases.
Emmaus Life Sciences global commercialisation senior vice-president George Sekulich stated: “The Omani marketing approval for Endari is an important step forward for us in the GCC region, where we have been working closely with the local authorities, physicians and patient groups to ensure access to this innovative treatment for sickle cell disease.
“We look forward to launching Endari commercially in Oman and expanding its availability to other countries in the region.”




