The Mundipharma network of independent associated companies has received European Commission (EC) approval for the use of Pelmeg (pegfilgrastim) for treatment of febrile neutropenia in adult cancer patients.
A pegfilgrastim biosimilar, Pelmeg is delivered subcutaneously to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients undergoing treatment of cytotoxic chemotherapy for malignancy. The exceptions are for chronic myeloid leukaemia and myelodysplastic syndromes.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pelmeg is the fourth biosimilar medicine to be commercialised by the Mundipharma network.
Mundipharma International biosimilars head Philippe Bastide said: “We hope this approval will significantly improve the lives of people who are affected by chemotherapy induced neutropenia and febrile neutropenia. The availability of this biosimilar represents an important opportunity to reduce healthcare costs while increasing access to an effective treatment option.”
The treatment was developed by Cinfa Biotech, which was acquired by Mundipharma network and announced in 2018.
Approval comes after a recommendation from the Committee for Medicinal Products for Human Use (CHMP) in September based on a regulatory submission of key biosimilarity data from analytical, biofunctional and clinical study comparisons for Pelmeg.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn all cases, the drug demonstrated comparable pharmacodynamics, pharmacokinetics, and immunogenicity to its reference product Neulasta, which was authorised by the EU in August 2002.
The information submitted and conclusions arrived at were sufficient to extrapolate the indication for Neulasta across to Pelmeg. Therefore, it is indicated in just the same way as subcutaneous (pre-filled syringe) Neulasta.
Pegfilgrastim is a pegylated version of granulocyte-colony stimulating factor (G-CSF) that stimulates bone marrow to produce more neutrophils, reducing the incidence of febrile neutropenia.
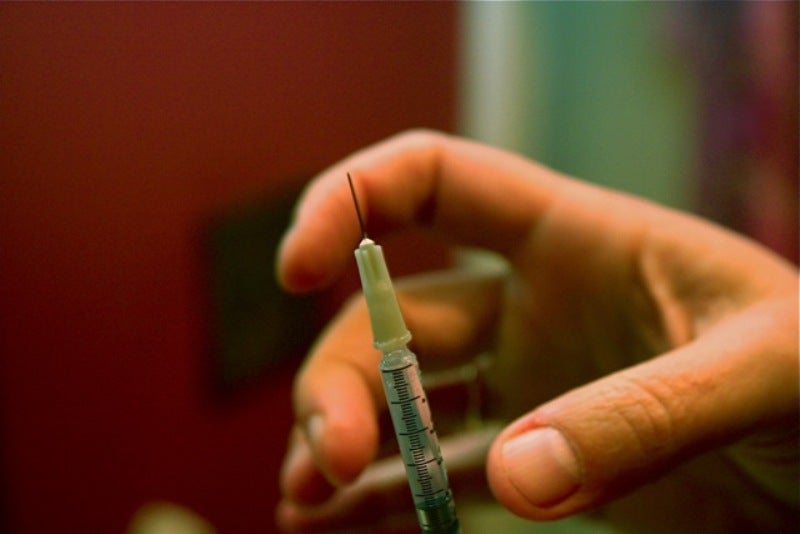
The treatment should be adminstered as a subcutaneous injection once for each chemotherapy cycle, at least 24 hours after cytotoxic chemotherapy.
People undergoing chemotherapy always stand at risk of having low levels of neutrophil, which is a type of white blood cell. Neutrophils boost the immune system and guard the body against infection.
Febrile neutropenia involves having a low level of neutrophils in the blood with a fever.


